Keywords
Feasibility, Introduction, Distribution, DMPA-SC, Community level, Zambia
This article is included in the International Conference on Family Planning gateway.
Feasibility, Introduction, Distribution, DMPA-SC, Community level, Zambia
Following key edits were made to the manuscript prompted by peer review comments:
1. Clarity in the Pilot description
2. Details of CBD profiles before the beginning of the pilot
3. Clarity on definition of a 'model facility' and 'adverse event'
4. Deletion and replacement of Figure 2 to show the age groups of clients who switched from other methods to DMPA-SC
5. Clarity on the results and conclusion for the determination that CBDs were safely able to administer DMPA-SC
6. Reviewed the manuscript for typos, as well as rewording of some sentences/paragraphs.
See the authors' detailed response to the review by Julie Hernandez
See the authors' detailed response to the review by Anthony K. Mbonye
See the authors' detailed response to the review by Jenny Liu
Over the last decade, the modern contraceptive prevalence rate (mCPR) in Zambia has increased from 25% to 45%. Despite this increase, unmet need for family planning (FP) stands at 21%1. Like other Sub-Saharan countries, injectable contraceptives are the most widely used modern method among currently married women in Zambia1–3. However, nearly one-third of the women who start on contraceptive injectables discontinue within the first year of use, partly due to access barriers such as long distances between a woman’s home and a health facility4. In response to the aforementioned, the Zambian Ministry of Health (MoH) introduced community-based distribution of short-term FP methods, including administering of injectable contraceptives by community-based distributors (CBDs)2.
Subcutaneous depot medroxyprogesterone acetate (DMPA-SC), brand name Sayana® Press, is a contraceptive injectable that uses a combination of low-dose DMPA in a pre-filed Uniject® system that eliminates preparation of needle and syringe5. With its unique Uniject® feature coupled with minimum level of training and supervision, DMPA-SC can be easily transported and safely administered by lower-level healthcare providers, including community health workers, and even self-administered by women themselves; thus, it may increase access to injectable contraceptives and improve method continuation, and ultimately address unmet need for FP4. Evidence from other similar pilots has shown that DMPA-SC is safe, acceptable and feasible in low- and middle-income country (LMIC) settings6–10.
In 2017, Society for Family Health – Zambia (SFH) a non-governmental organization in partnership with the Zambian MoH, Population Services International (PSI), ChildFund International and Development Aid from People to People (DAPP), conducted a pilot introduction of DMPA-SC in Zambia. This pilot was part of a five-year (2015–2020) United States Agency for International Development (USAID)-funded project titled Sexual and Reproductive Health for All Initiative (SARAI). The pilot aimed at understanding the feasibility of introduction community-based distribution of DMPA-SC in the community through use of CBDs.
Ethics approval (REF No. 006-12-13) to conduct the pilot was obtained from The University of Zambia Biomedical Research Ethics Committee (UNZAREC) to analyse routine client-level data on reproductive health. On consideration that SARAI was already being implemented in the pilot health facilities and that FP clients would be served as per routine by CBDs during project implantation, the ethics committee waived the need for participant consent. ‘Participant’ in this context refers to CBDs who were already volunteering for the project as well as clients who would receive DMPA-SC as an additional choice to the FP method mix that existed at the time.
The pilot was conducted from May 2017 to July 2017 in 29 SARAI-supported public health facilities across three districts (Kalulushi, Mafinga and Kawambwa). Voluntary FP services were routinely offered with established community-based distribution programs in which CBDs provided Intramuscular depot medroxyprogesterone acetate (DMPA-IM), oral pills and condoms.
At the time of the pilot, CBDs in Zambia were only authorized to distribute contraceptives other than condoms to returning clients only. This was a deliberate regulation by the Ministry of Health to ensure that only eligible clients, assessed by professional health workers were provided with appropriate FP services as new users. CBDs were part of community volunteers, who could read and write in English without necessarily having any medical or clinical background. They were provided with basic training and were observed for competence in provision of a particular health service within the catchment of their communities. In cases where CBDs encountered clients who had never used any modern contraception before, they provided general sexual and reproductive health counselling and refer such clients to a nearest health facility for eligibility assessment and possible commencement of FP services.
The pilot was implemented only in public health facilities that met the criteria of a “model facility” under SARAI. The project defined a model facility as one that encompasses the key components and systems needed at the community level to respond effectively to the family planning needs of the population. These systems included, skilled service providers; expanded method mix; client-centered care offering age appropriate information, outreach to vulnerable populations; supervision; mentoring for health care providers; youth and adolescent reproductive health services; community involvement through organized and trained community members who mostly are volunteers and data collection and analysis to track progress. Model facilities were selected because the project had already established necessary structures at facility and district levels to monitor and supervise community-based distribution of FP commodities. The CBD model under SARAI in Zambia is implemented in a way that CBDs operate in the peripherals of the catchment area of a health facility to distribute FP commodities and messaging. Each CBD is accountable to a nearby supervising facility and they submit monthly distribution and stock reports to the supervisors. All CBDs (163) in all model health facilities (29) supported by SARAI were selected to participate in the pilot.
A cascaded training approach was implemented which allowed introduction of DMPA-SC at all levels of the health system. In November 2016, prior to the pilot a 3-day training session for 25 national-level MoH master trainers drawn from all 10 provinces of Zambia was conducted. DMPA-SC master trainers with SRH expertise and experience drawn from key departments of the ministry of health were engaged to train 35 CBD supervisors drawn from three pilot implementing districts namely Kalulushi (13 supervisors), Kawambwa (12 supervisors) and Mafinga (10 supervisors). These supervisors included MoH health facility-based CBD supervisors and district-level supervisors with the following roles:
i. Post-training supervision to ensure CBDs attain proficiency
ii. Ensure CBDs adhere to injection safety standards
iii. Facilitate accurate data management and record keeping
iv. Commodity stock management
v. Monitoring and management of adverse events
There was 3 days of DMPA-SC training for 163 CBDs from implementing facilities, representing an average of five CBDs per facility, conducted. This training was in addition to the DMPA-IM training which was conducted prior to the pilot.
Each CBD was attached to a health facility, where he/she was to provide at least five DMPA-SC injections under supervision before being allowed to practice in the community. The standard MoH DMPA-SC injection step by step procedure was used to assess CBD competency in injection safety. Supervisors also conducted regular field-level supervision, which focused on data management and safe disposal of clinical waste. once a week for facility-based and twice a month for district health office-based supervisors. This supervision focused on safety of clients and CBDs during the administration of injections, data management and safe disposal of clinical waste. In order to comprehensively assess the pilot and draw lessons from the pilot, the National Family Planning Technical Working Group (NFPTWG) under the ministry of health conducted onsite monitoring visits to all implanting districts. The NFPTWG observed CBDs administering injections to clients, inspected storage sites for commodities stored by CBDs and assessed the clinical waste disposal process from the point of generation to the point of final disposal. The assessment measured safety of clients and CBDs based absence of injury during administration of injections, absence of AEs as well as safe disposal of clinical waste. The NFPTWG also conducted in-person interviews with CBD supervisors as well as clients to obtain their views on the pilot.
Stock consumption data obtained from the health information management system of the ministry of health for the three months (February-April 2017) that preceded the pilot showed that on average, each CBD administered 10 doses of DMPA-IM per month. Therefore, based on that precedence, each CBD was issued with 10 doses of DMPA-SC as initial post training stock. In order to avoid possible commodity stock out, each facility was given 30 doses as buffer stock. All CBDs were advised to re-order once 50% of stock on hand was used up. In addition, all CBDs were supplied with stock-tracking forms and were given lockable wooden boxes for storing commodities. Throughout the three months of the pilot, a total of 6,530 DMPA-SC doses were issued to implementing districts.
All trained CBDs were provided with supplies for waste management, including sharps boxes, buckets and bin liners. In line with MOH policy, CBDs ferried all waste generated during service provision to the nearest health facility for final disposal once the bin liners were three quarters full.
The pilot developed a framework for identifying, monitoring and real time reporting of AE’s. CBDs received training to identify, analyze and classify AEs in terms of nature and cause. An adverse event in this context refers to severe side effects from using the method. A referral system was established between the CBDs and the nearest health facility to link clients with suspected AEs.
CBDs captured client level data on clients receiving DMPA-SC doses using standard MoH registers, which had been adapted to include DMPA-SC as a new FP method. Demographic (i.e., age) and service information (i.e., client type and switching behaviours) for clients that voluntarily opted for reproductive health services, particularly DMPA-SC, were extracted from the registers and entered into Microsoft Excel software. For analysis, the study used data without personal identifying information for clients and were part of routine data collection during project implementation. IRB approval to use this level of data was obtained. Data analysis which included generation of descriptive statistics through tables, graphs cross-tabulations and frequencies was conducted using Statistical Package for Social Sciences (SPSS) version 21. CBDs characteristics data were collected by the project upon completion of the initial FP service provision training which was conducted prior to the pilot and were updated at the time of DMPA-SC training.
Of the 163 CBDs, 161 successfully completed the training; two CBDs discontinued due to personal reasons.
All the CBDs trained had previous training in FP counselling, distribution of oral pills and condoms and administration of DMPA-IM and were providing these services in the catchment of their communities prior to the pilot period. Below are the characteristics of the CBDs. Characteristics of CBDs that completed training are shown in Table 1.
| Variable | CBDs, N* |
|---|---|
| Total trained in DMPA-SC | 161 |
| Gender | |
| Male | 66 |
| Female | 95 |
| Education | |
| Primary | 21 |
| Secondary | 140 |
| Average age, years | 35 |
| Previous trained in DMPA-IM | 161 |
A total of 12,818 clients were provided with various FP methods/products during the pilot period as shown in Figure 1.
The majority of the clients (37%) were provided with male condoms while combined oral contraceptives at 5% accounted for the least method provided. DMPA-SC clients accounted for 16% of all the clients seen during the pilot period. Overall, clients that received injectable contraceptives (i.e., DMPA IM & SC) accounted for nearly half (i.e. 48%) of all the clients provided with FP methods/products during the pilot period.
Pilot results showed a 17% increase in the number of injectable DMPA (IM and SC) doses provided during the pilot period compared to the total for the three months preceding the pilot. Table 2 below compares FP provision before and during the DMPA-SC pilot.
| Variable | Prior to pilot (3 months) | Pilot period (3 months) |
|---|---|---|
| DMPA-SC doses | N/A | 2,100 |
| DMPA-IM doses | 5,284 | 4,090 |
| Total DMPA | 5,284 | 6,190 |
| # of CBDs | 163 | 161 |
| Avg. DMPA doses/3 months | 32 | 38 |
| Avg. DMPA doses/month | 11 | 13 |
Before the DMPA-SC pilot was introduced, each CBD distributed an average of 10 doses of injectable DMPA-IM per month. After the DMPA-SC pilot was introduced, each CBD provided an average of 13 doses of injectable DMPA per month (i.e., DMPA-SC=5, DMPA IM=8).
Out the 2,100 clients who were provided with DMPA-SC, 2,037 substituted their previous methods with DMPA-SC while the rest were new acceptors with no previous method. Figure 2 below shows the number of clients who switched from other FP methods to DMPA-SC disaggregated by age group.
Nearly half (43%) of the DMPA-SC clients were adolescents and young women below the age of 25. Figure 3 shows DMPA clients by age distribution.
The Majority of DMPA-SC clients (about 97%) were revisits on FP, while only 3% of DMPA-SC users were new acceptors, as indicated inn Figure 4.
Figure 5 shows the proportion of FP clients (re-visiting) that switched to DMPA-SC.
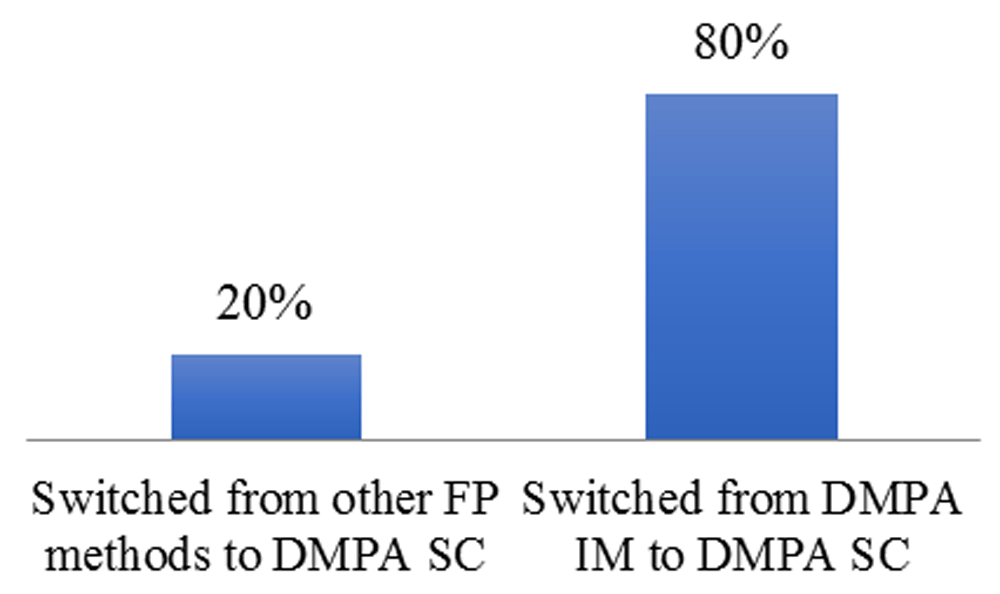
IM, intramuscular.
About 80% of FP clients switched from DMPA-IM to DMPA-SC, while 20% of the clients switched from other FP methods to DMPA-SC.
These findings provided useful insights to inform development of the national road map for the national scale-up of DMPA-SC in Zambia. This was the first study in Zambia to explore introduction of DMPA-SC in the community through use of CBDs. According to assessments carried out by CBD supervisors and the NFPTWG, trained CBDs were able to safely administer and appropriately store DMPA-SC, they were also able to dispose of resultant waste according to laid down procedures.
Similar to other DMPA-SC pilots conducted in Niger, Senegal and Uganda, this pilot revealed that nearly half DMPA-SC doses were administered to women younger than 25 years of age11. This was however a continuation of the similar trend for DMPA-IM even before the pilot. Each CBD distributed five doses of DMPA-SC doses per month on average compared to similar pilot in other countries (Senegal, Niger and Uganda), where each provider administered three doses per month11. Therefore, it is evident that DMPA-SC has the potential to provide an additional contraception choice for women especially the adolescents thereby reducing unintended pregnancies. Despite DMPA-SC being only available in the community, some facility-based CBD supervisors/FP providers in a few facilities included the method during FP counselling. Hence, new FP users who opted for DMPA-SC were assessed for eligibility and referred to CBDs for service provision. Due to policy differences between Zambia and other countries on FP service provision by CBDs and definition of ‘new acceptors of FP’, this finding contradicted with pilot results from other countries that showed a substantial number of women categorised as (“new users”) choosing DMPA-SC11.
The DMPA-SC Pilot demonstrated that CBDs can safely provide DMPA-SC within the existing family planning method mix. Secondly, the pilot demonstrated that DMPA-SC has potential to be a method of choice for adolescent and young women.
Open Science Framework: DMPA SC dataset. https://doi.org/10.17605/OSF.IO/X23FE12.
Underlying data are available in file Dataset.zip, which contains the following data:
DMPA SC aggregated dataset.xlsx (aggregated data from all locations).
KALULUSHI data.xlsx (underlying data from Kalulushi).
KAWAMBWA data.xlsx (underlying data from Kawambwa).
MAFINGA data (underlying data from Mafinga).
Figshare: CBD Profiles Dataset. https://doi.org/10.6084/m9.figshare.11829534.v113
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Contraceptives, DMPA-SC, adolescent health, sexual and reproductive health, health services delivery, behavioral economics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Sexual and Reproductive Health in Sub-Saharan Africa
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 04 Jun 20 |
read | read | |
|
Version 1 28 May 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)