Keywords
Feasibility, Introduction, Distribution, DMPA-SC, Community level, Zambia
This article is included in the International Conference on Family Planning gateway.
Feasibility, Introduction, Distribution, DMPA-SC, Community level, Zambia
Over the last decade, the modern contraceptive prevalence rate (mCPR) in Zambia has increased from 25% to 45%. Despite this increase, unmet need for family planning (FP) stands at 21%1. Like other Sub-Saharan countries, contraceptive injectables are the most widely used modern method among currently married women in Zambia1–3. However, nearly one-third of the women who start on contraceptive injectables discontinue within the first year of use, partly due to access barriers such as long distances between a woman’s home and a health facility4. In response to the aforementioned, Zambia Ministry of Health (MoH) introduced community-based distribution of short-term FP methods, including administering of contraceptive injectables by community-based distributors (CBDs)2.
Subcutaneous depot medroxyprogesterone acetate (DMPA-SC), brand name Sayana® Press, is a contraceptive injectable that uses a combination of low-dose DMPA in a pre-filed Uniject® system that eliminates preparation of needle and syringe4. With its unique Uniject® feature, DMPA-SC can be easily transported and safely administered by lower-level healthcare providers, including community health workers, and even self-administered by women themselves; thus, it may increase access to contraceptive injectables and improve method continuation, and ultimately address unmet need for FP5. Evidence has shown that DMPA-SC is safe, acceptable and feasible in low- and middle-income country (LMIC) settings6–10.
In 2017, Society for Family Health – Zambia (SFH) a non-governmental organization in partnership with Zambia MoH, Population Services International (PSI), ChildFund International and Development Aid from People to People (DAPP), conducted a pilot introduction of DMPA-SC in Zambia. This pilot was part of a five-year (2015–2020) United States Agency for International Development (USAID)-funded project titled Sexual and Reproductive Health for All Initiative (SARAI). The pilot aimed at understanding the feasibility of introduction of DMPA-SC in the community through use of CBDs.
Ethics approval (REF No. 006-12-13) was received from University of Zambia Biomedical Research Ethics Committee (UNZAREC) to analyze routine client-level data on reproductive health. The ethics committee waived the need for participant consent.
The pilot was conducted from May 2017 to July 2017 in 29 SARAI-supported public health facilities across three districts (Kalulushi, Mafinga and Kawambwa). Voluntary FP services are routinely offered with established and having established community-based distribution programs in which CBDs provided intramuscular DMPA (DMPA-IM), oral pills and condoms.
In Zambia, CBDs are not authorized to provide FP services to new users. CBDs refer new FP users to nearest health facility for medical assessment and subsequent commencement of services. CBDs are only authorized to attend to returning clients.
The pilot was implemented only in “model facilities” under SARAI. The project defined a model facility as one that encompasses the key components and systems needed at the community level to respond effectively to the FP needs of the population. These systems must include, skilled service providers; expanded method mix; client-centered care offering age appropriate information, outreach to vulnerable populations; supervision; mentoring for health care providers; youth and adolescent reproductive health services; community involvement and data collection and analysis to track progress. All CBDs (n=163) in all model public health facilities (n=29) supported by SARAI were selected to participate in the pilot.
A cascaded training approach was implemented which allowed introduction of DMPA-SC at all levels of the health system. In November 2016, prior to the pilot a 3-day training session for 25 national-level master trainers drawn from all 10 provinces of Zambia was conducted. DMPA-SC master trainers were engaged to train 35 CBD supervisors drawn from three pilot implementing districts namely Kalulushi (13 supervisors), Kawambwa (12 supervisors) and Mafinga (10 supervisors). These supervisors included MoH health facility-based CBD supervisors and district-level supervisors with the following roles:
i. Post-training supervision to ensure CBDs attain proficiency
ii. Ensure CBDs adhere to injection safety standards
iii. Facilitate accurate data management and record keeping
iv. Commodity stock management
v. Monitoring and management of adverse events
There was 3 days of training for 163 CBDs from implementing facilities, representing an average of five CBDs per facility, conducted.
Each CBD was attached to a health facility, where he/she was to provide at least five DMPA-SC injections under supervision before being allowed to practice in the community. The standard MoH injection supervision checklist was used to assess CBD competency in injection safety. Supervisors also conducted regular field-level supervision, which focused on data management and safe disposal of clinical waste.
Stock consumption data from the three months (February-April 2017) that preceded the pilot showed that on average each CBD administered 10 doses of DMPA-IM per month. Therefore, each CBD was given 10 doses of DMPA-SC following training. In order to avoid commodity stock out, each facility was given 30 doses as buffer stock. All CBDs were advised to re-order once 50% of stock on hand was used up. In addition, all CBDs were supplied with stock-tracking forms and were given lockable wooden boxes for storing commodities. A total of 6,530 DMPA-SC doses were given to implementing districts.
All trained CBDs were provided with supplies for waste management, including sharps boxes, buckets and bin liners. In line with MOH policy, CBDs ferried all waste generated during service provision to the nearest health facility for final disposal once the bin liners were 75% full.
The pilot developed a framework for identifying, monitoring and reporting of AE’s. CBDs received training to identify, analyze and classify AEs in terms of nature and cause. A referral system was established between the CBDs and the nearest health facility to link clients with suspected AEs.
CBDs captured client level data on clients receiving DMPA-SC doses using standard MoH registers, which had been adapted to include DMPA-SC as a new FP method. Demographic (i.e., age) and service information (i.e., client type and switching behaviours) for clients that voluntarily opted for reproductive health services, particularly DMPA-SC, was extracted from the registers and entered into Microsoft Excel software. The study used de-identified data for analysis that is part of routine data collection for which IRB approval was obtained. Data analysis involving generating of descriptive statistics through tables, graphs cross-tabulations and frequencies was conducted using Statistical Package for Social Sciences (SPSS) version 21.
Of the 163 CBDs, 161 successfully completed the training; two CBDs discontinued due to personal reasons.
All the CBDs trained had previous training in FP counselling, distribution of oral pills and condoms and administration of DMPA-IM. Below are the characteristics of the CBDs. Characteristics of CBDs that completed training are shown in Table 1.
| Variable | CBDs, N* |
|---|---|
| Total trained in DMPA-SC | 161 |
| Gender | |
| Male | 66 |
| Female | 95 |
| Education | |
| Primary | 21 |
| Secondary | 140 |
| Average age, years | 35 |
| Previous trained in DMPA-IM | 161 |
A total of 12,818 clients were provided with FP methods/products during the pilot period as shown in Figure 1.
The majority of the clients (37%) were provided with male condoms while combined oral contraceptives at 5% accounted for the least method provided. DMPA-SC clients accounted for 16% of all the clients seen during the pilot period. Overall, clients that received injectable contraceptives (i.e., DMPA IM & SC) accounted for nearly half (i.e. 48%) of all the clients provided with FP methods/products during the pilot period.
Pilot results showed a 17% increase in the number of injectable DMPA doses given after the introduction of DMPA-SC pilot. Table 2 below compares FP provision before and after DMPA-SC introduction.
| Variable | Prior to pilot (3 months) | Pilot period (3 months) |
|---|---|---|
| DMPA-SC doses | N/A | 2,100 |
| DMPA-IM doses | 5,284 | 4,090 |
| Total DMPA | 5,284 | 6,190 |
| # of CBDs | 163 | 161 |
| Avg. DMPA doses/3 months | 32 | 38 |
| Avg. DMPA doses/month | 11 | 13 |
Before the DMPA-SC pilot was introduced, each CBD gave an average of 11 doses of injectable DMPA-IM per month. After the DMPA-SC pilot was introduced, each CBD gave an average of 13 doses of injectable DMPA per month (i.e., DMPA-SC=5, DMPA IM=8).
A total of 6,190 injectable contraceptives were provided during the pilot period. DMPA-SC accounted for 34% (i.e., 2,100 DMPA-SC doses) of the total injectable contraceptives administered. Figure 2 below shows DMPA-SC doses provided as a proportion of total injectable contraceptives.
Nearly half (43%) of the DMPA-SC clients were adolescents and young women below the age of 25. Figure 3 shows DMPA clients by age distribution.
The Majority of DMPA-SC clients (about 97%) were revisits on FP, while only 3% of DMPA-SC users were new acceptors, as indicated inn Figure 4.
Figure 5 shows the proportion of FP clients (re-visiting) that switched to DMPA-SC.
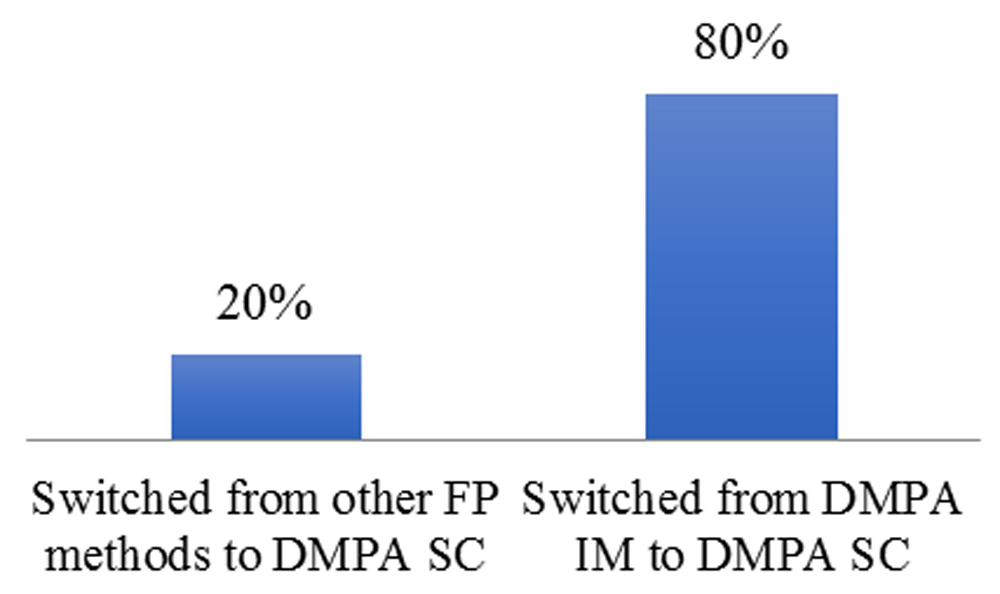
IM, intramuscular.
About 80% of FP clients switched from DMPA-IM to DMPA-SC, while 20% of the clients switched from other FP methods to DMPA-SC.
These findings provided useful insights to inform development of the national road map for the national scale-up of DMPA-SC in Zambia. This is the first study in Zambia to explore introduction of DMPA-SC in the community through use of CBDs. Trained CBDs were able to safely administer, appropriately store and dispose of DMPA-SC.
Similar to other DMPA-SC pilots conducted in Niger, Senegal and Uganda, this pilot revealed that nearly half DMPA-SC doses were administered to women younger than 25 years of age11. Each CBD gave five doses of DMPA-SC doses per month on average. The pilot performed relatively better than similar pilot in other countries (Senegal, Niger and Uganda), where each provider administered three doses per month11. Therefore, it is evident that DMPA-SC has the potential to reduce unmet need among women especially the adolescents thereby reducing unintended pregnancies. Despite DMPA-SC being only available in the community, some facility-based CBD supervisors/FP providers in a few facilities included the method during FP counselling. Hence, new FP users who opted for DMPA-SC were referred to CBDs after clinical assessment. Due to differences in policies on FP service provision by CBDs, this finding contradicted with pilot results from other countries that showed a substantial number of women using modern family planning for the first time (“new users”) choosing DMPA-SC11.
The DMPA-SC Pilot demonstrated that CBDs can safely provide DMPA-SC within the existing family planning method mix. Secondly, the pilot demonstrated that DMPA-SC has potential to reach underserved populations such as adolescent and young women.
Open Science Framework: DMPA SC dataset. https://doi.org/10.17605/OSF.IO/X23FE12.
Underlying data are available in file Dataset.zip, which contains the following data:
DMPA SC aggregated dataset.xlsx (aggregated data from all locations).
KALULUSHI data.xlsx (underlying data from Kalulushi).
KAWAMBWA data.xlsx (underlying data from Kawambwa).
MAFINGA data (underlying data from Mafinga).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
This study was funded by the United States Agency for International Development (USAID) under the terms of Award No. AID-611-A-15-00001 (Sexual and Reproductive Health for All Initiative - SARAI). The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of USAID. Publication of this study was sponsored by The Bill and Melinda Gates Foundation (Investment ID: OPP1181398).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Contraceptives, DMPA-SC, adolescent health, sexual and reproductive health, health services delivery, behavioral economics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Sexual and Reproductive Health in Sub-Saharan Africa
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 04 Jun 20 |
read | read | |
|
Version 1 28 May 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)