Keywords
Antimicrobial resistance, antimicrobial prescription, Clinical Decision Support System
The escalating threat of AMR demands a paradigm shift in antimicrobial prescribing practices. The application programming interface (API) is conceived as an advanced system, integrating artificial intelligence and machine learning, to optimize clinical decision-making in the context of antimicrobial therapy. This study outlines the development and evaluation of the software, emphasizing its potential impact on antimicrobial stewardship.
The API was meticulously constructed in two phases. In the initial phase, an algorithm leveraging decision flow, developed by a collaboration of information technology experts, infectious disease and microbiology specialists, was designed. This algorithm accounts for a comprehensive array of variables influencing antimicrobial treatment outcomes. Subsequently, a Machine Learning model was employed to assess the probability of success for each available antimicrobial drug. The second phase involved a rigorous evaluation through ten hypothetically described clinical cases, assessed independently by five infectious disease specialists (IDP team) in a double-blinded study. Results generated were then compared with the antimicrobial prescriptions made by the IDP team.
Utilizing the World Health Organization's AWaRe classification system as a benchmark, the API demonstrated a 50% prescription at both the Access and Watch categories, with a 0% allocation in the Reserve category. In comparison, the IDP team exhibited an 11.9% prescription in the Access, 73.9% at Watch, and 14.5% at Reserve category.
Despite potential disparities between expert opinions and the software, the proposed system, characterized by its conservative nature, holds promise in refining and validating clinical decisions. Moreover, the implementation of the API has the potential to mitigate selective pressure that contributes to antimicrobial resistance, thus fortifying antimicrobial stewardship practices.
Antimicrobial resistance, antimicrobial prescription, Clinical Decision Support System
Antimicrobial resistance (AMR) is a major global threat that impacts both high- income countries (HICs) and low- and middle-income countries (LMICs). Drivers of AMR, such as antibiotic misuse, poor sanitation, and a lack of infection prevention and control (IPC), disproportionately affect LMICs (Howard et al., 2014). Primary care is responsible for 80% to 90% of all antibiotics prescribed for humans (Pulcini et al., 2011). Despite the increasing use of antibiotics in LMICs over the past decade (Ergönül et al., 2016; Howard et al., 2014), primary care antibiotic prescribing in the African region falls significantly short of the World Health Organization's (WHO) reference targets (Rodríguez-González et al., 2019). Non-adherence to existing guidelines on antibiotic use, including delays and overuse, is linked to the behavior of patients and healthcare professionals (Lv et al., 2021; Peiffer-Smadja et al., 2020). Consequently, there is a pressing need to develop interventions to improve antibiotic prescribing in primary care in response to the global threat of AMR (Peiffer-Smadja et al., 2020).
The issue of antimicrobial resistance (AMR) is an increasingly serious and pressing matter that poses a significant threat to global health and health institutions and is an enormous burden on society and the economy. While the use of antimicrobials is often necessary for suspected infections, empiric drug administration or definitive therapy for infections with a defined etiology based on laboratory results is getting more challenging every day. It is important to note that most prescriptions are made by physicians, who, despite having a degree in medical care, may not have a comprehensive understanding of antimicrobial therapy, including the optimal spectrum, dosage, posology, duration, and, especially evidence surrounding the continuous evolution on AMR (Howard et al., 2014; Pulcini et al., 2011). As the prevalence of AMR increases, the likelihood of initiating inappropriate empiric therapy also rises, leading to fewer treatment alternatives and ultimately impacting the clinical outcomes of patients.
A multicentric study conducted in 2019 estimates 4.95 million deaths associated with AMR and 1.27 million attributed to AMR. Even before the COVID-19 pandemics, there was an evident rise in AMR, especially in Brazil and Latin America. The first wave of the COVID-19 pandemic led to an estimated increase of 216.4 million doses of non-pediatric antibiotics. Azithromycin alone accounted for 38.0 million doses (Kanan et al., 2023).
In the context of AMR, the use of artificial intelligence methods, such as machine learning and other multivariate statistical methods, is increasing worldwide (Rodríguez-González et al., 2019; Peiffer-Smadja et al., 2020). These methods prove to be useful in contexts of large numbers of variables, promoting the practice of evidence-based medicine (Jiang et al., 2017). Clinical decision support systems (CDSS) use the characteristics of a patient to generate assessments and recommendations to support decisions made by doctors on treating bacterial infections more assertively (Weernink et al., 2014). In addition, the prioritization of lower-spectrum antimicrobials is observed, lowering selective pressure towards antimicrobials and improving the quality of health care (Carracedo-Martinez et al., 2019; Holstiege et al., 2014).
While there is a plethora of literature on computerized clinical decision support systems (CDSS) for antimicrobial prescribing, many gaps still exist. Currently, most CDSS are rule-based, adhering strictly to guidelines and policies, which can make them inflexible and difficult to put into practice. To combat AMR effectively, it is necessary to conduct regular, efficient, and effective clinical reviews of all suboptimal antimicrobial prescriptions and guidelines, as well as up-to-date epidemiology on AMR (Howard et al., 2014; Pulcini et al., 2011).
Considering the threat of antimicrobial resistance (AMR) and the emerging advanced applications of CDSS, a new system, developed by microbiologists, medicine students, pharmacists, information technology and infectious disease specialists has been developed. This system utilizes an algorithm based on antimicrobial pharmacokinetics and pharmacodynamics, local antimicrobial susceptibility testing epidemiology (BR-GLASS, Pillonetto et al., 2021), and patient demographics to enhance better decision-making. To evaluate the impact of the API, we conducted a retrospective observational analysis comparing its outputs to the prescription of a team of infectious disease physicians (IDPs).
The system developed in this study is based on the MCDA (Multi-Criteria Decision Analysis) model. The MCDA model is a set of various analytical techniques used to assist in the decision-making process in the context of multiple conflicting criteria.
MCDA (Multi-Criteria Decision Analysis) involves establishing the decision context, identifying the alternatives to be evaluated, identifying the criteria to be assessed, identifying the criteria for assessing the consequences of each decision, organizing the criteria by grouping them at both high-level and lower-level hierarchies, and evaluating the expected performance of each alternative concerning the criteria, through scores.
MCDA also involves describing the consequences of each alternative, classifying the alternatives according to the criteria, checking the consistency of scores for each criterion, adding weights to each criterion to reflect its relative importance for the decision, calculating the weighted scores at each level in the hierarchy, and calculating the overall weighted scores. Finally, the results are organized, and a sensitivity analysis is done by the tool. The study was conducted in two phases (Table 1).
An artificial intelligence system using decision flow was developed by information technology experts with the support of infectious disease specialists (physicians, pharmacists, and microbiologists), considering all variables that could affect the outcome of an antimicrobial treatment.
Initially, the system requests the patient's baseline and demographic data, such as type of infection (outpatient/community or hospital-acquired); patient localization (hospital/city/state, as available); age; gender; pregnancy and breast-feeding status (if pregnant); drug allergies; renal function (creatinine and creatinine clearance); sepsis risk (calculated or presumed by the physician); site of infection, and related comorbidities.
The software can also run some microbiological parameters, if available, such as clinical sample; Gram stain results, bacterial identification, and susceptibility testing. The epidemiological data is obtained through compiled results of antimicrobial sensitivity testing from the BR-GLASS program, consisting of over 32,000 bacterial isolates (up to 2021), from five different hospitals in three cities (Pillonetto et al., 2021).
Following the data, the software employs a Machine Learning model to assess the probability of success for each antimicrobial available, utilizing the patient's clinical data and the antimicrobial susceptibility testing database (BR-GLASS). The Machine Learning algorithm used for this process is the Naive Bayes. This algorithm has proven to be more efficient than other complex algorithms, such as Logistic Regression and Random Forest. Naive Bayes is a fast algorithm that requires minimal processing and provides traceability of results. In comparative tests, the results between Naive Bayes and other algorithms were not significant.
After this step, the algorithm calculates the average probability and its respective confidence interval for the hypothetical success of each medication. Next, the results obtained for each medication are adjusted to a list of specifications that are not in the BR-GLASS database and, therefore, not evaluated by Naive Bayes, such as sepsis risk, kidney function, allergies, comorbidities, and pregnancy or lactation.
This list of rules and constraints is based on guidelines developed by pharmaceutical experts, infectious disease specialists, and microbiologists. The list utilizes weights to adjust the best medications for the case. For example, if an antimicrobial has the highest probability of success, but the patient has a specific comorbidity, the tool will consider this data and use it on the final recommendation for the patient. Currently, the system employs 20 rules with varying levels of constraints for each medication.
Additionally, after drug ranking, the system provides some parameters on pharmacological therapy, such as dose, route of administration, dosing, and therapy duration, and in the near future it will display some pharmacokinetic data, such as plasma protein binding rate, and alternative routes or duration of drug administration. The acceptability rate and relative costs of pharmacological therapy will also be considered.
At this stage, ten clinical cases were hypothetically described and evaluated by five specialists (well-trained infectious disease physicians). These professionals were responsible for choosing the top three antimicrobials they would prescribe for each case presented. The study was conducted in a double-blind manner to eliminate any potential biases.
The system was fed with the clinical case information, and the generated results were compatible with the antimicrobial prescription. Then, the results generated by the system were compared with the ones obtained from the specialists. Subsequently, the specialist versus the API results were compared using the World Health Organization's classification of antibiotics (Access, Watch, Reserve [AWaRe]), which emphasizes the appropriate use of each antimicrobial, using a conservative prescription rule.
The API is set to assist physicians in prescribing antibiotics, integrating with the BRGlass system through an API. It is designed to be used in real cases, providing support based on population data, local information, and antimicrobial resistance sensitivity.
Integration with BRGlass: a system that stores data on antimicrobial resistance. This allows the application to access updated and relevant information for antibiotic prescription. By utilizing data collected from hospitals and organized into a national database, it is possible to outline the sensitivity profile of microorganisms isolated in cultures, as well as assist in empirical prescription. The data from BRGlass enable inference of the likely pathogen in the infection indicated by the prescribing physician.
Probabilistic Analysis: The application performs a probabilistic analysis of the available data, considering the efficacy of antibiotics in different clinical scenarios. This includes the analysis of medical occurrences, and the prescription of antibiotics based on historical data and resistance patterns embedded in the algorithm, reviewed by experts in antimicrobial resistance, and following the latest guidelines recommended for antibiotic prescription.
Improvements in Recommendation:
Granularity Weighting: The system adjusts recommendations based on data granularity, allowing for more precise personalization of prescriptions.
Surrogates: It uses substitute data to fill information gaps, ensuring that recommendations are based on the maximum available data.
Sepsis Risk Calculator: It considers the risk of sepsis, adjusting recommendations for cases where there is widespread infection and imminent life risk, favoring safer prescriptions even when they must be made empirically.
Application of Restrictions: The application applies restrictions based on weighting and sensitivity, considering specific patient data such as allergies, administration route, conditions like diabetes, pregnancy, lactation, renal function, and sepsis risk.
Data Filtering: It uses heuristics to filter data from medical occurrences, ensuring that only relevant information is considered in the analysis. This includes outpatient data (age, sex, origin, affected area) and laboratory data (bacterial group, family, and genus).
Antibiotic Estimation: The application calculates the efficacy of antibiotics based on equivalent occurrences, ensuring that the minimum data sample is considered for a reliable recommendation.
Application Example: In a case of community-acquired cystitis in an adult woman, the application analyzes data such as age, sex, origin, and laboratory results (when available) to recommend the most effective antibiotic according to the location and its respective sensitivity profile.
Ethics and consent not required for the study
The API application was designed for both IOS and Android platforms. To utilize the software, users initiate the process by inputting relevant data pertaining to the clinical case in question (Figure 1)
Based on this, the software calculates the result using a built-in algorithm and the epidemiological data recorded in the BR-GLASS database, ranking at least three antimicrobial prescription recommendations, along with their respective percentages of susceptibility and the number of samples analyzed (Figure 2).
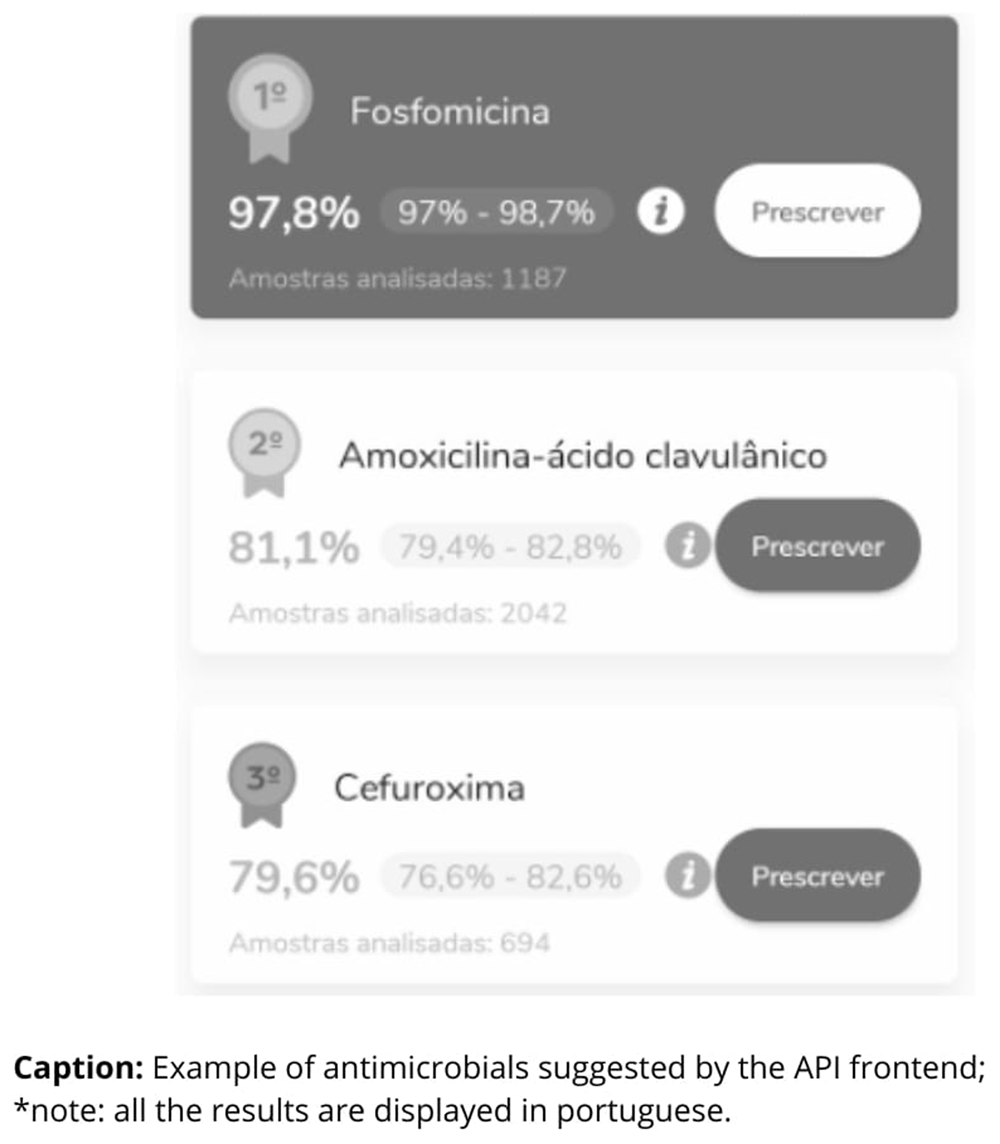
When selecting the antimicrobial, the software provides standard dose options, as well as the possibility of calculating a specific dose. The three first antimicrobial options for each clinical case were compared to the three first options of the antimicrobial suggested by the team of experts (IDPs).
To compare the results, we used the WHO's AWaRe classification system (see Figure 3), which aims to promote the rational and responsible use of antimicrobials, categorizing them into three categories: Access, Watch, and Reserve. The Access category includes first-line antimicrobials for treating common communities with a lower risk of resistance; the Watch category includes second- line or higher-risk antimicrobials that should be used cautiously and monitored.
Healthcare-associated infections (HAIs) are directly related to high levels of morbidity and mortality. In Europe, it is estimated that HAIs cause an additional 16 million hospitalization days, lead to 37,000 deaths annually, and generate a financial impact of around 7 billion euros. In the United States of America (USA), the economic impact is even more significant, from 28.4 to 33.8 billion dollars annually (WHO, 2021).
Antimicrobials play a crucial role in fighting HAIs. However, inadequate exposure of bacteria to these drugs, often resulting from indiscriminate prescriptions and consumption, leads to the selection of resistant microorganisms. It is estimated that 30% of antibiotic prescriptions are unnecessary (WHO, 2021).
According to the World Health Organization, the fight against antimicrobial resistance is a global priority and a shared responsibility for all. It is estimated that by 2050, antimicrobial resistance will become one of the leading causes of mortality, responsible for 10 million deaths worldwide (O'neill, 2014; WHO, 2019a; WHO, 2021).
Furthermore, WHO also encourages research and development of new antimicrobials, as well as the promotion of emergency prevention and control measures, such as proper hand hygiene and vaccination. Other actions include strengthening antimicrobial resistance surveillance systems and improving access to quality antibiotics, especially in low and middle-income countries. Combating antimicrobial resistance is a global responsibility that requires a coordinated and multidisciplinary approach (WHO, 2019b; WHO, 2021)
Clinical decision support systems (CDSS) are an important tool to assist healthcare professionals in making decisions and choosing the most appropriate treatment for each patient. They utilize individual patient data, such as health history and test results, to provide personalized recommendations for healthcare professionals. This can help improve the accuracy of diagnosis and reduce the risk of prescription errors, as well as promote more rational use of antimicrobials (Peiffer-Smadja et al., 2020).
CDSS can be classified into two types: those that provide unsolicited information, which are automatic alerts or reminders for the physician about possible drug interactions, adverse drug reactions, or dosage recommendations, and those that provide requested information, which are diagnostic or treatment support systems that help the physician make more informed choices. Both types can help improve healthcare quality and reduce medical errors (Peiffer-Smadja et al., 2020).
Similar studies in other hospitals and countries have also shown a significant reduction in antimicrobial use after the implementation of CDSS, suggesting that this approach may be effective in promoting rational antimicrobial use globally. Additionally, CDSS can help reduce the risk of antimicrobial resistance, as more accurate and appropriate prescription of antimicrobials can limit unnecessary exposure of microorganisms to antimicrobials.
It is important to observe that CDSS is not a universal solution for all types of infections and clinical conditions. The implementation of these systems must be evaluated and adapted to the specific needs of each institution and patient. Additionally, studies emphasize that CDSS should be managed using guideline-based intervention and other measures to maximize positive impact on prescription quality and clinical outcomes.
The antibiotic compendium entitled WHO AWaRe (Access, Watch, Reserve) provides concise and evidence-based guidelines for the choice, dose, route of administration, and duration of treatment for over 30 common clinical infections in both children and adults. The information in this document supports the antibiotic recommendations found in the WHO Model List of Essential Medicines and the Model List of Essential Medicines for Children. WHO AWaRe is accompanied by concise infographics for each infection for adults and children, providing a quick reference guide for healthcare professionals in the clinical setting.
The fact that the API suggests more conservative choices of antimicrobials according to the WHO's AWaRe classification can be beneficial in preventing antimicrobial resistance, as excessive and inadequate use of antibiotics is one of the main causes of bacterial resistance development. However, healthcare professionals' adherence to the use of SMART-CDSS may be challenging, especially when the software's suggestions differ from their personal choices or there are inconsistencies in the epidemiological database. Additionally, healthcare professionals must have access to adequate training and support for using the system to ensure its effectiveness in clinical practice.
This classification separates antibiotics into three groups based on their therapeutic potential and possible impact on therapy and antimicrobial resistance. Group 1 - "Access" comprises drugs considered as the first or second choice for treating common infections, inducing lower resistance, and that have the characteristic of being widely accessible. Group 2 - "Alert" includes those medications indicated only for a specific group of patients with well-defined diseases and syndromes, requiring continuous monitoring of their use. Finally, group 3 - "Reserved" encompasses antibiotics that should be used as a last resort to treat bacterial infections refractory to multiple drugs.
The use of antibiotics belonging to the more conservative classes ("Access") contributes to the reduction of bacteria resistant to these drugs, and the WHO advises that 60% of antimicrobial prescriptions be composed of them.
In our research, the result of the API, which indicates the prescription of antimicrobials, showed an agreement of 40% compared to the IDPs. However, the program's choices of antimicrobials were more cautious, in line with the AWaRe classification.
Adequate integration of surveillance, monitoring, and decision support systems based on epidemiological data can serve as a crucial tool in enhancing healthcare quality and facilitating efficient data management in healthcare, as stated by the WHO in 2021. However, as mentioned, it is imperative to ensure that these systems are regularly updated with precise and reliable epidemiological data to ensure the effectiveness of the clinical decision support system. Using technologies which enables real-time integration of local data, can provide accurate and up-to-date recommendations to healthcare professionals.
According to the systematic review conducted by Tokgoz et al. (2023) which aimed to identify the factors that affect the implementation of decision support systems for antibiotic prescription, most of the factors are related to the technological and organizational aspects of implementing these systems. On the other hand, the study conducted by Biezen et al. (2020) which evaluated the usability and feasibility of a clinical decision support tool (CDST), highlighted the importance of resources such as easy navigation, clear and useful guideline content, adaptation to clinical workflow, and integration into the electronic medical record.
However, this study identified other difficulties in implementing and synthesizing the software, such as incomplete and inconsistent data from the epidemiological database for some clinical samples, such as insufficient samples from the lower respiratory tract in community infections. Additionally, the study anticipates future challenges, such as the possible lack of adherence of clinicians to the use of the system, as well as the divergence of opinions among specialists regarding the best therapy indicated for each patient due to the conservative nature of the AWaRe prescribing system.
It is important to consider that implementing decision support systems for antibiotic prescription is a complex process involving technological and organizational factors that can affect the success of their implementation and use by healthcare professionals. Furthermore, the quality of the epidemiological data and guidelines used in developing the system is fundamental for its effectiveness. Usability and adaptation to the clinical workflow are also important aspects to be considered in the implementation of these systems. Therefore, it needs to be a joint effort by developers, managers, and healthcare professionals to ensure the success of implementing these systems and, consequently, improve the quality of patient care.
The API was developed to support healthcare professionals in clinical decision-making regarding antimicrobial treatment. It uses local epidemiological data to present treatment recommendations. Although there may be disagreements between experts and the system, due to its conservative nature, the software has the potential to improve and validate clinical decisions, as well as reduce selective pressure towards antimicrobial resistance.
Repository: Github - The API source code, available at: https://github.com/3778/smart-cdss-api.git (Smart-cdss-api, 2024)
The code is available under MIT License
MIT License
Copyright (c) 2019 Ricardo Giglio & Robson Zagre Junior
Permission is hereby granted, free of charge, to any person obtaining a copy of this software and associated documentation files (the "Software"), to deal in the Software without restriction, including limitation without the rights to use, copy, modify, merge, publish, distribute, sublicense, and/or sell copies of the Software, and to permit persons to whom the Software is furnished to do so, subject to the following conditions:
The above copyright notice and this permission notice shall be included in all copies or substantial portions of the Software.
THE SOFTWARE IS PROVIDED "AS IS", WITHOUT WARRANTY OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO THE WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NONINFRINGEMENT. IN NO EVENT SHALL THE AUTHORS OR COPYRIGHT HOLDERS BE LIABLE FOR ANY CLAIM, DAMAGES OR OTHER LIABILITY, WHETHER IN AN ACTION OF CONTRACT, TORT OR OTHERWISE, ARISING FROM, OUT OF OR IN CONNECTION WITH THE SOFTWARE OR THE USE OR OTHER DEALINGS IN THE SOFTWARE.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new software tool clearly explained?
Yes
Is the description of the software tool technically sound?
Partly
Are sufficient details of the code, methods and analysis (if applicable) provided to allow replication of the software development and its use by others?
Partly
Is sufficient information provided to allow interpretation of the expected output datasets and any results generated using the tool?
Partly
Are the conclusions about the tool and its performance adequately supported by the findings presented in the article?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomedical informatics, clinical decision support systems, artificial intelligence in healthcare, antimicrobial stewardship, electronic health records
Is the rationale for developing the new software tool clearly explained?
Yes
Is the description of the software tool technically sound?
Yes
Are sufficient details of the code, methods and analysis (if applicable) provided to allow replication of the software development and its use by others?
Partly
Is sufficient information provided to allow interpretation of the expected output datasets and any results generated using the tool?
Partly
Are the conclusions about the tool and its performance adequately supported by the findings presented in the article?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Antimicrobial Resistance and Stewardship
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 11 Mar 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)