Keywords
Malaria, nanopore, P. falciparum, surveillance, antimicrobial resistance, AMR, vaccine, amplicon sequencing
Malaria causes around 250 million cases and over 600,000 deaths annually, with the heaviest burden falling on young children living in sub-Saharan Africa. Molecular surveillance of Plasmodium parasites and Anopheles mosquito vectors are key components of effective malaria control decision-making. Previously, we have designed and implemented a nanopore-based workflow for targeted P. falciparum molecular surveillance in Ghana, which we call DRAG1 (drug resistance + antigen multiplex PCR). Here, we describe an updated and expanded multiplex assay (‘DRAG2’) with additional amplicon targets that incorporate more antimalarial drug resistance markers, the polymorphic surface antigen merozoite surface protein 2 (msp2), and the 18S ribosomal RNA (rRNA) gene for Plasmodium species detection. We describe the performance of the DRAG2 assay over a range of parasitaemias and sample types (venous blood and dried blood spots), with suggested systems of quality control including the use of synthetic plasmids for positive controls and recommended coverage thresholds. The plasmids are highly economical, and engineered to include both ‘test’ single nucleotide polymorphisms (SNPs), such as known drug resistance markers, and ‘control’ SNPs, which are not found in nature and thus signal contamination if detected in clinical samples. We provide standard operating procedures (SOPs) for use by teams aiming to implement the assay in their laboratory. In summary, we describe an updated nanopore-based method for malaria molecular surveillance, including detailed consideration of quality control processes and SOPs. These are important steps in the transition from research tool to diagnostic assay, which will require further testing in endemic settings and regulatory processes and approvals.
Malaria is a major cause of disease and death globally, especially for young children living in Africa. Affected countries aim to reduce the spread of the malaria parasite, which is transmitted by mosquitoes, and to treat infected people using drugs. However, the parasites can develop resistance to the drugs, making them less effective. Newly approved malaria vaccines are also being deployed at scale. It is therefore important to monitor parasites for the development and spread of drug resistance and for any changes relevant to the vaccine, to ensure treatment outcomes remain optimal. This study describes a laboratory method for monitoring malaria parasites suitable for use in tropical areas where malaria is common. Starting from a blood sample taken from an infected person, targeted sections of malaria parasite DNA are amplified and sequenced using nanopore technology. The genes analysed provide information on drug resistance, the current vaccine target, and which malaria species are present. The study also describes laboratory practices to ensure high quality standards are maintained consistently. In summary, this study reports a method for monitoring malaria parasites using a streamlined DNA sequencing workflow; further work can assess its performance in malaria zones and develop protocols suitable for clinical use.
Malaria, nanopore, P. falciparum, surveillance, antimicrobial resistance, AMR, vaccine, amplicon sequencing
Malaria exacts a huge toll on human health globally, particularly for young children living in sub-Saharan Africa. There were 249 million malaria cases and 608,000 deaths in 2022 across 85 countries.1 The number of cases in 2022 increased by five million compared to 2021. 95% of cases and deaths were in sub-Saharan Africa, and around four in five malaria deaths were children under five years old. Threats to malaria control include antimalarial drug resistance, deletions in the diagnostic target genes hrp2 and hrp3, vector insecticide resistance, and the spread of invasive vector species. Continuous political will and investment to sustain and intensify control efforts is essential. A changing climate may further contribute to fluctuations in transmission dynamics in the coming years.
Despite these challenges, there are multiple new interventions being added to the antimalarial armamentarium, including novel antimalarial drugs, long-acting monoclonal antibodies, the expansion of molecular systems of surveillance in endemic countries, and several vaccines. Currently, two malaria vaccines are recommended by the World Health Organization (WHO), RTS, S and R21, which both target circumsporozoite protein (PfCSP) in the most virulent malaria parasite species, Plasmodium falciparum. Parasite populations are therefore being placed under a multitude of intense selection pressures, creating the ecological conditions that may drive the evolution of antimalarial drug resistance or possibly vaccine escape mutants. In this dynamic context, continuous surveillance of parasite populations forms a critical component of malaria control, allowing national malaria control programmes (NMCPs) to deploy their resources most effectively and respond quickly to heritable changes in the parasite that confer fitness against interventions.2
Nanopore sequencing has been increasingly used to generate genetic data on Plasmodium parasites, including in endemic countries, such as detecting antimalarial drug resistance markers, variation in the csp gene sequence, hrp2/3 gene deletions, and population dynamics.3–6 Nanopore technology offers advantages including relatively low up-front costs, ‘real-time’ sequencing, portability, scalability, and long sequence reads. Moreover, use of Oxford Nanopore Technologies (ONT) has expanded in malaria endemic countries during the Covid-19 pandemic, in efforts to increase global SARS-CoV-2 genomic surveillance.7 However, significant challenges to implementation persist, including optimising sampling strategy, the low quality and quantity of parasite DNA generally retrieved from clinical samples, slow procurement processes in many endemic countries, and bioinformatics capacity (reviewed in Ref. 8).
Previously, we have developed and implemented a nanopore-based multiplex amplicon assay for P. falciparum genomic surveillance in Ghana.5 This assay had targets within six genes, five for monitoring antimalarial drug resistance (chloroquine resistance transporter (crt), dihydrofolate reductase (dhfr), dihydropteroate synthase (dhps), multidrug resistance protein 1 (mdr1), and kelch13), and one for vaccine target surveillance (csp). We refer to this assay as ‘DRAG1’ (drug resistance + antigen multiplex PCR). Here, we expand on this work by adding additional targets to the multiplex PCR, producing a new assay we refer to as ‘DRAG2’. We describe assay performance, quality control processes including coverage thresholds and use of synthetic plasmids as positive controls, and standard operating procedures (SOPs) for assay implementation.
The DRAG2 multiplex PCR builds on our previous iteration of the assay (‘DRAG1’) by adding additional amplicons for the drug resistance genes crt and mdr1, the full-length merozoite surface protein 2 (msp2) gene, and a target within the small subunit of ribosomal rRNA genes that can distinguish between Plasmodium species. A summary of the amplicons is shown in Table 1 and Extended data (all supplementary materials for this project are freely available at: https://doi.org/10.6084/m9.figshare.28539320.v1).27 We divided the assay into two separate multiplex reactions, which we refer to as DRAG2-A and DRAG2-B. This reduced the risk of primer interactions and increased target specificity. In addition to the drug resistance markers targeted in DRAG1, the expanded DRAG2 assay includes C-terminal sections of crt and mdr1 ( Table 2). Full-length msp2 was included due to its polymorphism, with potential to act as an approximate indicator of multiplicity of infection (MOI) i.e. infections with multiple P. falciparum clones. While the assay is intended as a tool for P. falciparum molecular surveillance, the 18S rRNA gene target was included to detect mixed species infections or unintended non- falciparum malaria samples; further details are provided below. The amplicon targets were designed to be more similar to each-other in size compared with DRAG1, to make sequencing coverage more even (average and standard deviation (SD) of amplicon sizes in the 3D7 reference genome for DRAG1 and DRAG2 assays are 586bp (SD 303bp) vs. 668bp (SD 166bp), respectively).
The assay is divided into two multiplex PCR mixtures, DRAG2-A and DRAG2-B. Amplicon size is relative to the 3D7 reference genome. ‘New’ refers to whether the primer sequences are new since the DRAG1 assay (already published), or were already included in DRAG1. Primer sequences are also shown in Supplementary Table 2.
Red text indicates targets added in DRAG2 that are not included in DRAG1. Both DRAG1 and DRAG2 assays also include the region of kelch13 in which artemisinin resistance mutations have been identified and the vaccine target csp.
Primer design followed a similar process to,5 using primer3 software9–11 to generate candidate primer sequences, followed by in silico checks for primer interactions with ThermoFisher Multiple Primer Analyzer,12 and in vitro testing of primer combinations to identify high-performing combinations based on PCR product inspection by agarose gel electrophoresis and nanopore sequencing to determine gene target coverage profiles.
For species detection, we chose the 18S rRNA gene because (1) this is already well described as a target for Plasmodium species detection13–15; (2) 16S and18S gene amplicon sequencing is already well used and well understood in clinical microbiology practice as a molecular method of microbial identification16; (3) the multiple copies of 18S rRNA in the Plasmodium genome effectively increases the amount of template DNA present for amplification, which may increase sensitivity; (4) we found improved multiplex PCR performance if all primers targeted the nuclear genome, rather than adding mitochondrial targets for species detection to the same PCR reaction as nuclear genome targets, perhaps due to different AT-content and extremely different ploidy between the mitochondrial and nuclear genomes. 16S and 18S rRNA genes have sections of high sequence conservation interspersed with regions that vary between species, making them well suited for microbial identification via primers that target the more conserved regions. This approach is also well suited to the long sequence reads possible with ONT.
Primer sequences (except for the 18S rRNA primers) were selected in conserved regions across high-quality P. falciparum genomes spanning wide geographic origins17 based on multiple sequence alignments, and then screened for variants in the Pf7 MalariaGEN P. falciparum Community Project data resource.18 For 18S rRNA primer design, publicly available 18S rRNA sequences from P. falciparum, P. vivax, P. ovale-curtisi , P. ovale-wallikeri , and P. knowlesi were aligned to identify conserved regions between all species for primer placement, containing regions that varied between species within the amplified sequence. The 18S rRNA primer pair chosen was not expected to amplify a product from the human genome (this was subsequently confirmed using a human gDNA sample).
Positive and negative controls are essential components of quality assurance in diagnostic microbiology. Negative controls should undergo the same end-to-end processes as test samples, including using the same reagents. This should identify reagent contamination, which could lead to false inferences by assigning contaminant genotypes to clinical samples (contamination risk is reduced by strict separation of pre- and post- PCR laboratory locations, pipettes and tips). Positive controls are required (1) to identify where problems are arising in a laboratory protocol; and (2) to confirm that the assay should have been able to detect a true positive (for example, if all test samples are negative). Positive control material could also form part of internal and external quality assurance processes; for example, by testing samples with known resistance genotypes and ensuring concordance, and investigating any discrepancies. Using P. falciparum genomic DNA as positive controls has several disadvantages, including: (1) a risk of clinical sample contamination, leading to false conclusions; (2) it can be slow and resource-intensive to culture P. falciparum parasites in vitro to generate gDNA material, requiring human erythrocytes and laboratory equipment; (3) it may be difficult or impossible to obtain parasites and/or genetic material that contain specific mutations to test assay performance, such as kelch13 mutations found in non-culture adapted parasite lines; (4) non- falciparum malaria species are difficult to culture and obtain genetic material, making positive controls for Plasmodium species identification challenging.
We addressed these challenges by designing a system of synthetic plasmids for use as positive controls. The plasmids were purchased from a commercial company (GenScript) using a pUC57 vector background. Each plasmid contained a Plasmodium gene section targeted in the DRAG1 and DRAG2 PCRs, including the non- falciparum 18S rRNA gene sequences Extended data; Supplementary Tables 3-4).27 Each plasmid insert sequence was based on the 3D7 reference genome (or the corresponding non- falciparum reference genomes for 18S rRNA genes), but modified in two ways: (1) ‘test SNPs’ were added, consisting of known drug resistance mutations or other genetic changes the assay should detect; and (2) ‘control SNPs’, which were SNPs never found in nature (based on screening the Pf7 MalariaGEN data resource) and which result in amino acid substitutions with BLOSUM62 scores of -2 or below, suggesting they are biologically unlikely. Full details of test and control SNPs for the plasmid inserts are shown in Extended data; Supplementary Table 5),27 and the fasta sequences are provided in Extended data.27 At least two test SNPs and two control SNPs were added per insert sequence. It is extremely unlikely that two control SNPs would genuinely occur in a clinical sample; therefore, these SNPs act as markers of positive control contamination in clinical samples, and would facilitate contamination being quantified highly accurately from sequence read counts. Quality assurance systems could monitor for control contamination levels, with cut-offs to trigger investigation based on absolute read counts, relative read counts, and trends over time.
200ug of each plasmid arrived from GenScript at a concentration of 1ug/ul in 200ul TE buffer. Each plasmid was diluted by a factor of 100 (to 10ng/ul) and combined accounting for their size to achieve the same number of moles per plasmid within the mixture. Inserts were not separated from the plasmid backbones. Five plasmid mixtures were created: mixture 1 contained plasmids with inserts spanning amplicon targets of dhfr, dhps, mdr1, kelch13, csp, and three P. falciparum 18S rRNA sequences (representing the amplicon diversity present in the five P. falciparum 18S rRNA genes present in the 3D7 reference genome). The remaining four mixtures contained mixture 1, plus: 2) P. malariae 18S, 3) P. ovale 18S, 4) a mixture of P. malariae and P. ovale 18S, and 5) P. vivax 18S, to represent mixed-species Plasmodium infections. Serial dilutions were created for plasmid mixture 1, starting at 10ng/ul, to: 5ng/ul, 2.5ng/ul, 1ng/ul, and 0.5ng/ul. 2ul mixture 1 was added into the DRAG2 multiplex PCR assay (total reaction volume 25ul), i.e. total plasmid mixture masses added per 25ul PCR reaction were: 20ng, 10ng, 5ng, 2ng, and 1ng. From gel electrophoresis inspection, the first three concentrations (20ng, 10ng, and 5ng) produced all the expected bands with high intensity; some bands were faded at the lower concentrations. We opted to use the 20ng mixture going forwards, but would expect similar results down to at least 5ng. The plasmid cost is less than US$0.1 (10 US cents) per PCR reaction, and the 200ug starting quantity would be sufficient for at least 100,000 PCRs. Figure 1 shows the DRAG1, DRAG2-A and DRAG2-B multiplex PCR products visualised by agarose gel electrophoresis using genomic DNA and the plasmid mixtures (20ng plasmid mass).
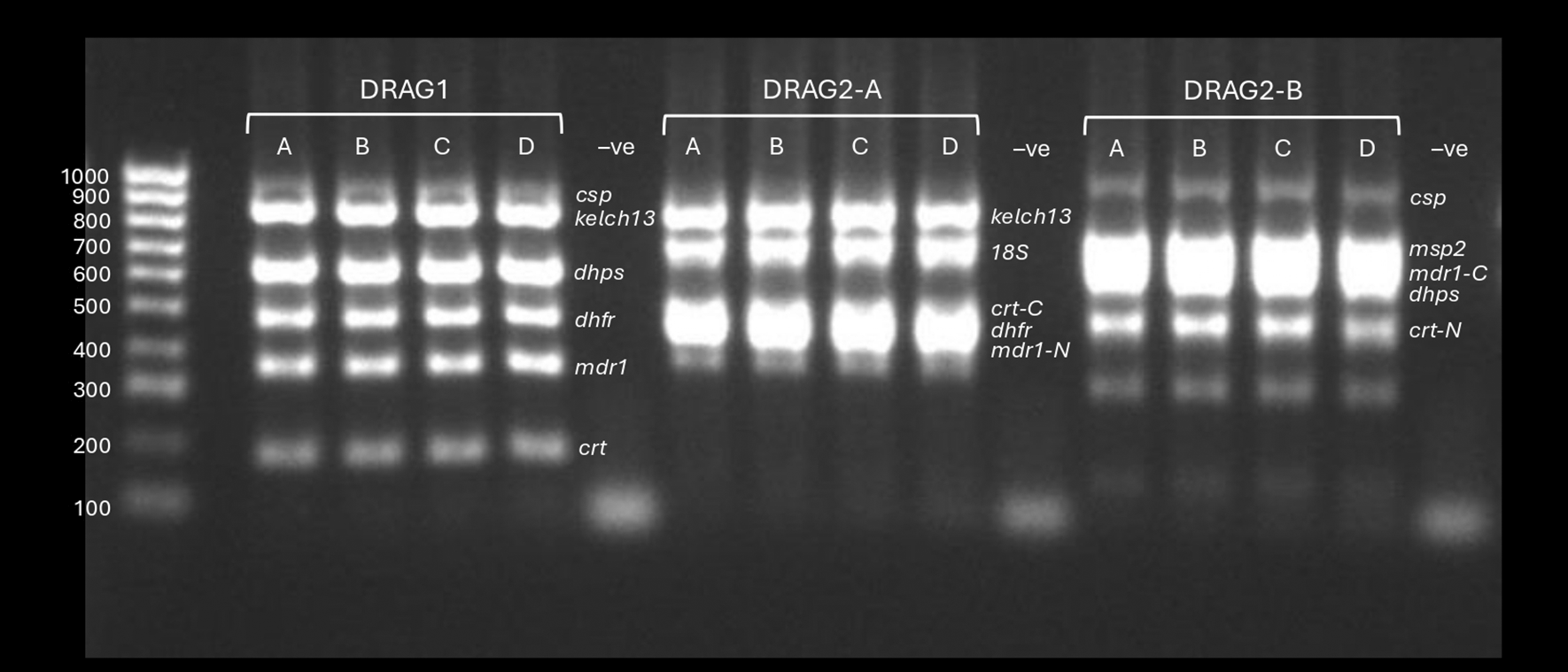
DRAG1, DRAG2-A and DRAG2-B multiplex PCRs visualised by 2% agarose gel electrophoresis. Samples A-C use plasmid mixtures containing the inserts targeted in the assays at different concentrations; sample D is P. falciparum genomic DNA (laboratory clone DD2). The plasmid mixtures for samples A-C were, respectively: Mixture 1 containing the drug resistance and csp target regions plus three P. falciparum 18S rRNA sequences, Mixture 2 with added P. malariae 18S rRNA sequences, and Mixture 3 with added P. ovale 18S rRNA. All plasmids had been diluted to 10ng/ul with 2ul of plasmid mixtures added per PCR reaction (20ng total). ‘-ve’ refers to nuclease free water template negative controls.
Finally, the Oxford Nanopore Technologies (ONT) kit 14 native barcoding protocol (SQK-NBD114) also includes ‘spike DNA’, that functions as a positive control to confirm nanopore sequencing has worked for each barcode (separately from the PCRs, tested by the plasmid positive controls).
This study tested blood samples from clinical malaria patients recruited in Ghana, collected in Accra and in Navrongo in the Upper East Region. The samples were reported on previously in,5 with a small number of additional samples from the same collection reported here. Patients were recruited into the study from LEKMA Hospital in Accra, and from community clinics and the War Memorial Hospital in Navrongo. Participants had symptoms compatible with malaria and tested positive for P. falciparum malaria by rapid diagnostic testing and microscopy. Participation required informed consent, and a detailed information sheet was provided. Samples collected included both leucodepleted venous blood (VB) and dried blood spots (DBS). Leucodepletion was performed by centrifugation and Buffy coat removal. Sample processing is described in more detail in.5
To test the 18S amplicon for Plasmodium species identification, samples of P. vivax, P. ovale and P. malariae were selected based on microscopy findings. Five P. vivax samples were tested from patients in Laos. These samples were collected by the GenRe-Mekong study,19 which performs genomic surveillance in five provinces of southern Laos. Collections were coordinated by the Center for Malariology, Parasitology, and Entomology (CMPE), Vientiane, Lao PDR, and Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit (LOMRU), Vientiane, Lao PDR. The study is based at the Mahidol-Oxford Tropical Medicine Research Unit (MORU), Mahidol University, Bangkok. The P. ovale and P. malariae samples were collected from patients who presented with fever at two health facilities in Lomé, Togo, as part of an ongoing study of host-parasite interactions led by author LNA.
As in,5 DNA extraction for the leucodepleted VB samples was undertaken using New England Labs Monarch High Molecular Weight (HMW) DNA extraction kit for cells and blood (T3050) according to the manufacturer protocol, and a subset of samples were extracted using the QIAmp DNA blood mini kit (51106) according to manufacturer instructions. DBS samples had been transferred from Ghana to the Wellcome Sanger Institute (WSI) and DNA was extracted using the QIAamp Investigator Biorobot kit on the Qiagen Biorobot Universal instrument. Further details are provided in Ref. 5.
The non- falciparum samples (P. vivax, P. malariae and P. ovale) were DBS samples extracted using the QIAmp DNA blood mini kit (51106) according to manufacturer instructions.
In summary, while this study made use of samples that had been extracted using HMW DNA extraction kits, both the DRAG1 and DRAG2 assays also work from DNA extracted using ‘standard’ DNA extraction methods such as the QIAmp DNA blood mini kit. This is consistent with the fact that the largest amplicon fragment in either DRAG1 or DRAG2 assays is csp, around 1Kb in size.
We have produced an end-to-end protocol for the ‘wet lab’ components of the workflow, including details on DNA extraction, PCR, sample clean-up and ONT library preparation and sequencing, which is available in Extended data.27
The two multiplex PCR reaction mixtures (DRAG2-A and DRAG2-B) are shown in Table 3, and the PCR conditions are shown in Table 4.
| DRAG2A | x1 run (μL) | DRAG2B | x1 run (μL) |
|---|---|---|---|
| 5X HiFi Buffer | 5 | 5X HiFi Buffer | 5 |
| 10mM dNTP | 0.75 | 10mM dNTP | 0.75 |
| kelch13- Forward | 0.6 | csp-Forward | 0.75 |
| kelch13- Reverse | 0.6 | csp -Reverse | 0.75 |
| 18S -Forward | 0.85 | msp2-Foward | 0.75 |
| 18S- Reverse | 0.85 | msp2-Reverse | 0.75 |
| crt-C- Forward | 0.75 | mdr1-C- Forward | 0.6 |
| crt-C -Reverse | 0.75 | mdr1-C-Reverse | 0.6 |
| dhfr-Forward | 0.75 | dhps-Forward | 0.6 |
| dhfr-Reverse | 0.75 | dhps-Reverse | 0.6 |
| mdr1-N-Forward | 0.5 | crt-N -Forward | 0.6 |
| mdr1-N-Reverse | 0.5 | crt-N -Reverse | 0.6 |
| Template DNA* | * | Template DNA * | * |
| HiFi enzyme | 0.5 | HiFi enzyme | 0.5 |
| Water (NFW) | Add to final vol | Water (NFW) | Add to final vol |
| Total | 25 | Total | 25 |
* Volume of template DNA added depends on sample type (DBS, VB or plasmid control mixture). For DBS, typically we had concentrations after elution of 1-7 ng/ul, and we added 15ul per 25ul PCR reaction for both DRAG2-A and DRAG2-B. For leucodepleted VB, we had concentrations after elution of 2-500ng/ul and added 4ul sample per 25ul PCR reaction. The plasmid mixtures had been diluted to 10ng/ul and we added 2ul per 25ul PCR reaction.
All nanopore sequencing described in this study was undertaken using ONT kit 14 chemistry with R10.4.1 flow cells using the MinION Mk1B. The native barcoding kit SQK-NBD114.24 was used for library preparation, as per manufacturer instructions. We have also tested the 96-plex native barcoding protocol using SQK-NBD114.96 as per manufacturer instructions and achieved similar results, at higher throughput and therefore reduced cost per sample. MinKNOW software (version 23.11.2 – 24.02.08) was used, running on commercially available high-performance ‘gaming’ laptop computers with an NVIDIA RTX Graphics Processing Unit (GPU) of specification 3080 or higher, as in Ref. 5. For a batch of 24 samples running on a new MinION mk1b R10.4.1 flow cell, sequencing was stopped after 14 hours. Real-time base calling was performed via the MinKNOW software interface using the dorado base caller (version 7.2.11-7.3.11) on super accuracy mode.
Fastq files produced by sequencing and real-time base calling were processed using the nano-rave Nextflow pipeline (nanopore rapid analysis and variant explorer), as in Ref. 5. Nano-rave is available open-access via GitHub at: https://github.com/sanger-pathogens/nano-rave . Briefly, nano-rave takes fastq files as input, maps the reads to reference sequences provided by the user using minimap2,20 and performs variant calling with a user-selected choice of callers. In this study, the reference sequences provided were the coding sequences of genes targeted in the DRAG assays from the P. falciparum 3D7 reference genome, and the Clair3 variant caller was used with diploid genotypes.21 An example nano-rave command line is shown below:
nextflow run main.nf --sequencing_manifest./seq_manifest_name.csv --reference_manifest./ref_manifest_name.csv --variant_caller clair3 --clair3_args “--model_path/opt/models/r941_prom_sup_g5014 --no_phasing_for_fa --include_all_ctgs” --results_dir/output_directory -with-trace
Nano-rave generates three output files: coverage statistics using BEDTools, variant call files (vcfs) produced by the variant caller, and quality control (QC) metrics. Coverage data for each amplicon were used to assess assay performance and are presented in Figures 2-3. vcf files were processed using a custom R script as in Ref. 5, with the following modification: the medaka-haploid 22 variant caller was used in,5 which infers haploid genotypes. We used the default diploid genotyping function of Clair3. Heterozygous genotypes (representing mixed infections) were then converted to haploid calls by using the majority allele (i.e. the allele present in >51% of reads).
For investigation of Plasmodium species, reads were mapped to the full-length genomes of P. falciparum 3D7 (Pf3D7_01_v3 version 2020-09-01), P. malariae (PmUG01_01_v1 version 2020-09-01), P. vivax (PvP01_01_v2 version 2020-09-01) and P. ovale (LT594505 version 2018-06-15) using minimap2. Mapped read pileups were inspected using the Integrative Genomics Viewer (IGV) software tool.23 Bam files were manipulated using samtools.24,25 The Bam-readcount tool was used to calculate read counts at each position. Plots were produced using pandas, seaborn and matplotlib.
As in Ref. 5, ethical approval for sample collection in Ghana was granted through the PAMGEN study (ethics approval ID: NHRCIRB343, obtained from the NHRC Institutional Review Board 31/05/2019), and via the EGSAT study (ethics ID: ECBAS030/21–22, approved by the College of Basic and Applied Sciences Ethics Review Committee, University of Ghana, 20/12/2024). Ethical approval for the GenRe-Mekong study, from which the P. vivax samples were derived (collected in 2022 in Attapeu Province, Laos), was granted from the National Ethics Committee for Health Research (NECHR) of the Health Ministry of the Lao PDR, issued on 18th August 2016. The Oxford Tropical Research Ethics Committee (OxTREC) approval for the study is dated 3rd August 2016, amended 1st August 2018. Ethical approval for the study from which the P. ovale and P. malariae samples were collected in Togo was obtained from Comité de Bioéthique pour la Recherche en Santé (CBRS), under the Ministry of Health, Public Hygiene, and Universal Access to Care (Opinion N° 045/2023/CBRS), 02/11/2023. Written informed consent was documented prior to enrolling patients into all the above studies. Approval was granted by the Wellcome Sanger Institute Research Ethics Committee and the study complied with all relevant ethical and research regulations.
We tested the DRAG2 assay on 122 P. falciparum clinical samples collected from Ghana; 34 were dried blood spots (DBS) and 88 were leucodepleted venous blood (VB). 95 samples were already reported in Ref. 5, with 27 new samples from the same study collections. The samples encompassed a wide range of parasitaemias as is commonly seen in clinical collections, from 1 parasite per 200 white blood cells (WBC) to 6378 parasites per 200 WBC, or from the limit of microscopy positivity to approximately 320,000 parasites per ul of blood. Amplicon coverage for the DRAG2 assay was more even than for DRAG1. Median coverage across all DRAG2 amplicon targets, including those that failed quality control (QC) filtering, was 10,727x (interquartile range (IQR), 3,745-24,774); including only QC-pass amplicons, median coverage was 11,781x (IQR, 4938-26,925). Coverage was lower for samples in the 0-40 parasites per 200 WBC range from DBS samples ( Figure 2), suggesting that 40 parasites per 200 WBC (or approximately 2000 parasites per ul of blood, or 0.1% infected red blood cells (RBCs)) may be a pragmatic cut-off for the DRAG2 assay from DBS samples. Coverage remained high for VB samples, even at the lowest parasitaemias tested.
We analysed DRAG2 amplicon coverage for clinical samples, positive controls, and negative controls ( Figure 3). We established a pragmatic cut-off for clinical samples to be at least 7.5x the coverage of negative controls for each amplicon in the run to pass QC filters, on the basis that this would “pass” all the positive controls tested across multiple sequencing runs. We also used an absolute coverage threshold of 50x per amplicon, and if a sample has three or more amplicons that fail QC (out of the 10 amplicons in the assay) then the whole sample was failed. The poorest performing amplicon was for the dhps gene target, with 18 fails, whereas csp and msp2 amplicons had no fails. Median coverage for each amplicon is shown in Extended data; Supplementary Table 6.27 Seven samples (6%) were deemed to have failed QC on the basis of three or more failed amplicon targets, six DBS and one VB sample. These samples had low parasitaemias – the six DBS had median parasitaemia of 3 parasites per 200 WBC (range 1-27), and the VB sample had 232 parasites per 200 WBC. As a further QC step, each variant needed at least 50x coverage for valid genotyping. While coverage was fairly even over each amplicon, it declined at the C-terminal end of the crt-C amplicon, likely due to primer slippage in a nearby AT-rich intron.
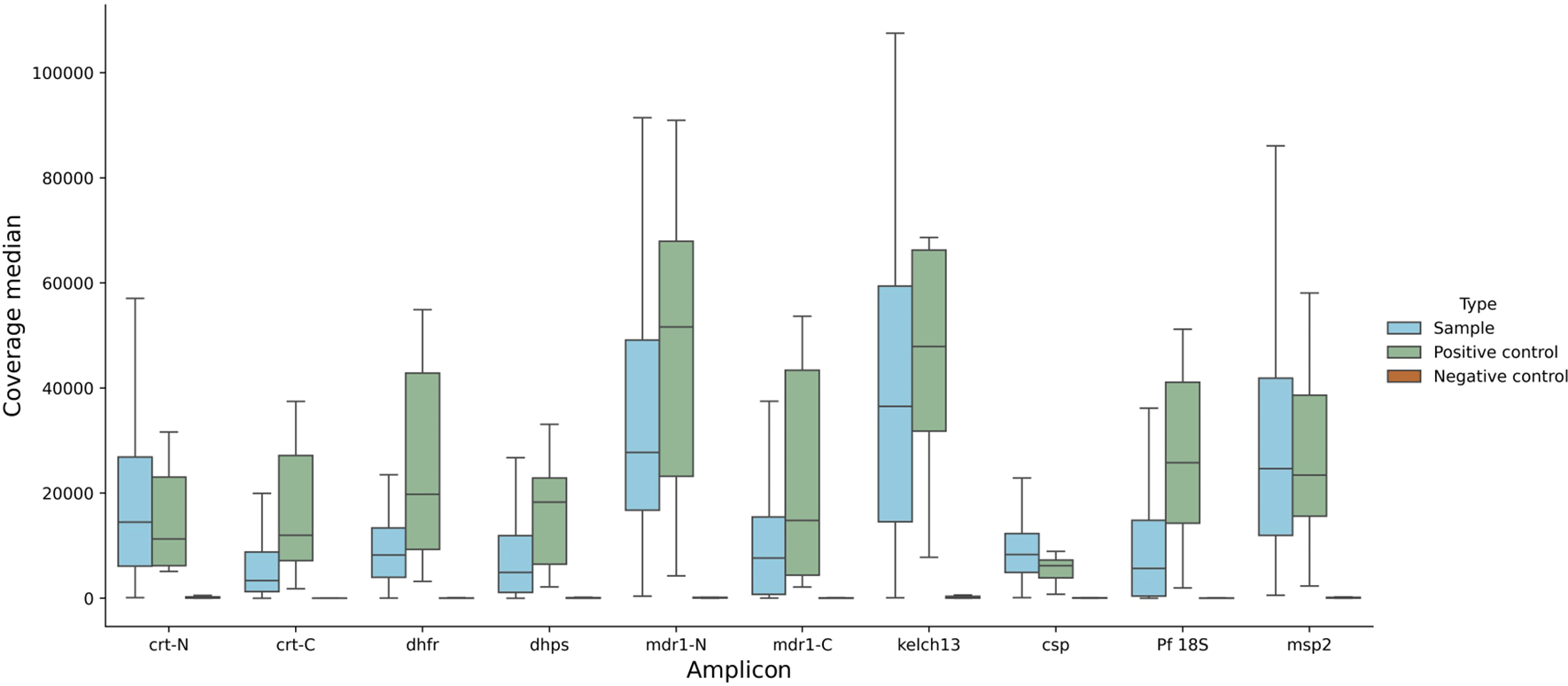
Data across all parasitaemias tested for DBS and VB samples, for positive controls, negative controls, and clinical samples. Note that coverage appears lower for msp2, because reads were mapped competitively against both 3D7 and DD2 msp2 sequences, which encompass the main two allelic forms of msp2. The coverage shown for all amplicons depicts reads mapped to 3D7 sequences.
Drug resistance marker frequencies were calculated from the variants called from the amplicon sequence data ( Table 5). These were consistent with the frequencies calculated from the DRAG1 assay reported in Ref. 5. No mutations associated with artemisinin partial resistance were identified in kelch13.
Out of 122 samples, seven were excluded due to ≥3 failed amplicons, giving N=116 samples. Individual amplicons were also excluded (QC criteria described in main text), so each amplicon has a different denominator. DRAG2 counts were tested for significance against expected proportions from the DRAG1 assay using Binomial exact test. Samples used for the DRAG1 and DRAG2 assay overlapped but with some samples unique to each assay. The SNP column shows the position of the SNP in the gene coding sequence in the 3D7 reference genome, and the base pair substitution. *P<0.05, **with Bonferroni correction for multiple comparisons, 0.05/11: <0.005. No frequency comparisons were statistically significantly different between the DRAG1 and DRAG2 assays.
We tested the 18S rRNA target for Plasmodium species detection in two ways. First, we used plasmid mixtures that included 18S rRNA sequences from P. falciparum (Pf ), plus different combinations of P. malariae (Pm), P. ovale (Po), and P. vivax (Pv) 18S rRNA sequences. We identified SNPs within the 18S rRNA sequences that were only found in their respective species, and counted the number of sequence reads containing these ‘species-determining SNPs’ Extended data; Supplementary Table 7).27 As expected, for ‘pure’ P. falciparum plasmids, 99.15% reads mapping to the 3D7 18S rRNA sequence contained the Pf-determining SNPs (reflecting the accuracy of PCR and ONT sequencing). The mixtures contained approximately 2:1 ratios of Pf to non- Pf plasmids, and accordingly the proportion of reads mapping to the 3D7 rRNA gene that contained the Pf-determining SNPs for these mixtures ranged from 62-81%, with the vast majority of remaining reads containing SNPs corresponding to the appropriate non- Pf species ( Figure 4A). Next, we tested clinical DBS samples available from other studies that had been confirmed by microscopy as being Pf (from Ghana), Pm (Togo), Po (Togo), and Pv (Laos) ( Figure 4B), using only the 18S primers in singleplex reactions. Over 90% of reads mapping to the Pf 3D7 18S rRNA gene contained species-determining SNPs for the Pf, Pm and Pv samples (provided in Extended data; Supplementary Table 8)).27 For the Po sample, 79% reads contained the Po-determining SNP and 21% contained a Pf-determining SNP; this may represent a mixed-species infection with low-level Pf parasitaemia. Reads were also mapped to their corresponding species reference genomes and inspected manually in IGV, and BLAST to confirm they were indeed the correct identity, matching the microscopy. These data suggest that the 18S rRNA gene target can be used as part of the DRAG2 multiplex panel to explore the prevalence of mixed Plasmodium species infections and the relative abundance of the non- Pf species from clinical samples; and also functions as a stand-alone assay for Plasmodium species determination.
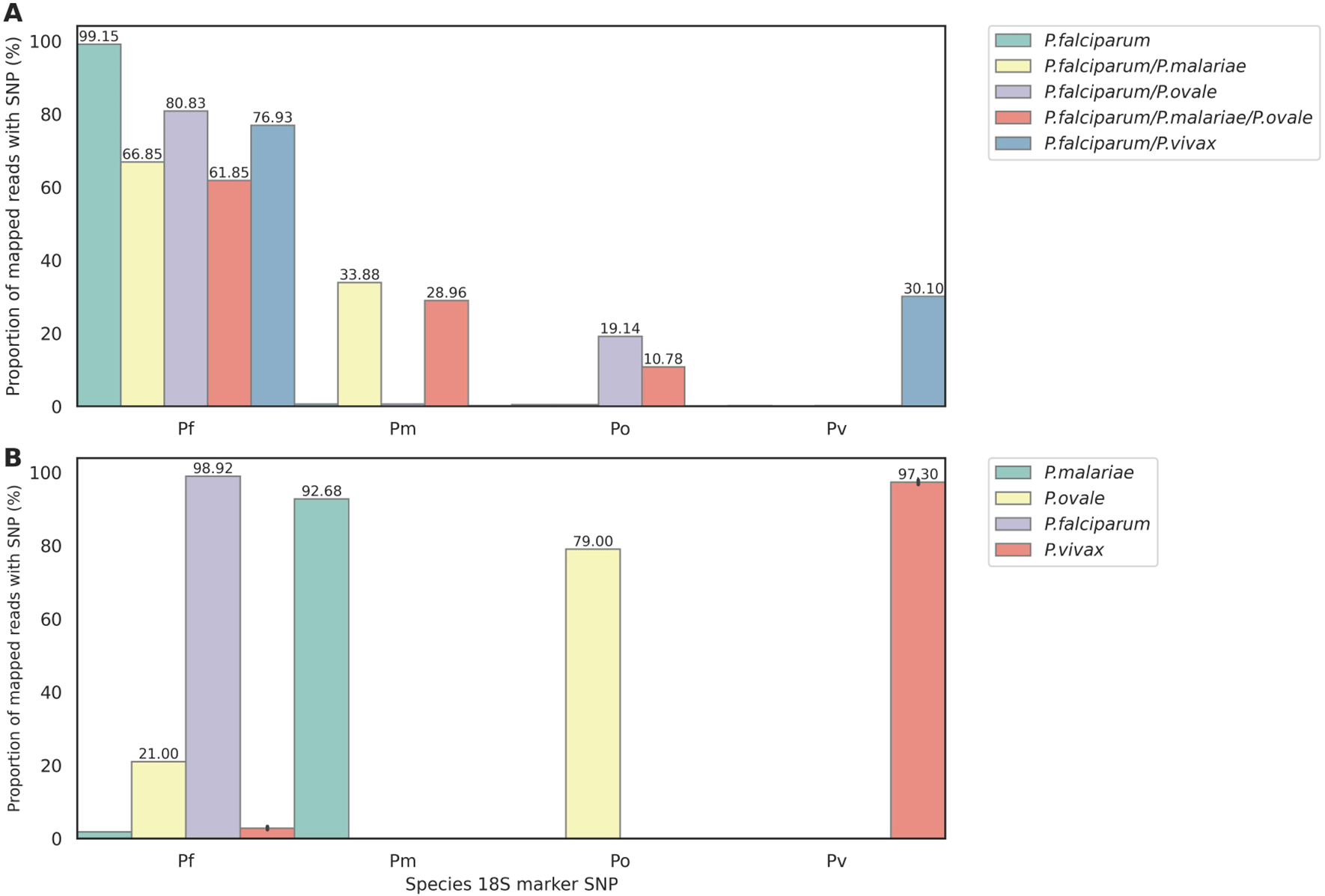
Reads were first mapped to the Pf 18S rRNA sequence. (B) Species-determining SNP prevalence in clinical DBS: one P. falciparum (Ghana), one P. malariae (Togo), one P. ovale (Togo), and five P. vivax (Laos).
This study describes a method for malaria parasite molecular surveillance using targeted nanopore sequencing and a method to use plasmids as synthetic positive and negative controls for assay performance and quality controls. We build on our previous work,5 expanding the targets in the multiplex PCR to include additional regions of the drug resistance genes crt and mdr1, full-length msp2, and a section of the 18S rRNA gene to support Plasmodium species identification. We have tested the method on blood samples from clinical malaria patients over a range of parasitaemias, using both leucodepleted venous blood (VB) and dried blood spots (DBS). The method does not involve a selective whole genome amplification (sWGA) step and instead works directly from DNA extracted from clinical samples, saving time and resources. The method is effective for VB down to the lowest microscopy-positive parasitaemias, though for DBS samples the coverage drops at parasitaemias below around 40 parasites per 200 white blood cells (the same coverage threshold commonly used for sWGA26). The assay can detect key drug resistance markers in the genes crt, dhfr, dhps, and mdr1; mutations in the propeller domain of kelch13 that are associated with artemisinin partial resistance; diversity in the csp vaccine target and the polymorphic surface antigen msp2 – which may be used as an approximate indicator of multiplicity of infection; and uses the 18S rRNA gene to identify Plasmodium species. This straightforward workflow could therefore further enhance the ability of researchers in endemic settings to provide useful data to guide national malaria control programme decision-making. The full protocol is provided in Extended data,27 presented as a Standard Operating Procedure (SOP) for laboratory use.
We have used plasmids as positive control template DNA, engineering both ‘test SNPs’ and ‘control SNPs’ into the insert sequences. These plasmids can be used to confirm that the assay detects specific genetic markers of interest, such as drug resistance mutations (test SNPs), and simultaneously enable early detection of contamination of clinical samples by plasmid DNA (via the control SNPs). Because the control SNPs are extremely unlikely to occur in nature, and multiple SNPs are unlikely to arise by chance from sequencing error, contamination can be assessed on a read-by-read basis using this method, enabling absolute and relative thresholds and temporal trend data to trigger laboratory investigation for potential contamination. The plasmids are highly cost-effective, at around $0.1 (10 US cents) per PCR reaction, removing the need for gDNA from cultured parasites as positive controls. We also explored coverage cut-offs for quality control (QC) filtering, by determining the fold-changes in coverage for negative controls compared with clinical samples and plasmid positive controls. We suggest that for each amplicon, clinical samples should have at least 7.5x the coverage of the negative control to pass QC filters, in addition to an absolute threshold (for example, 50x coverage per amplicon). Another advantage is the ability to combine plasmids at precisely defined ratios, for example, to mimic mixed Plasmodium species infections and P. falciparum mixed clone infections. These features make plasmids well suited to assay quality assurance processes.
This study has several limitations. The DRAG2 assay has been tested using a previously collected sample set from Ghana5 and a small number of samples with non- falciparum malaria species, which did not include P. knowlesi. The assay should be implemented as a practical workflow in laboratories based in malaria endemic countries to assess ‘real world’ performance on a larger sample size.
In conclusion, we have developed an expanded version of our previous multiplex assay for malaria molecular surveillance (MMS) using targeted nanopore sequencing; we provide assay validation data over a range of clinical sample types and parasitaemias, and described the use of engineered plasmid vectors for use as controls. A detailed SOP is provided in Extended data,27 intended for use by laboratories adopting this workflow for MMS. Protocol standardisation, use of positive and negative controls, and systems of internal and external quality assurance (IQA and EQA) are essential components of assay quality assurance, which must be developed for malaria molecular surveillance to transition from a research tool to clinical and public health applications.
Nanopore sequence data for all samples analysed in this study is available via the European Nucleotide Archive (ENA), with sample codes provided in Supplementary Table 9. Prior to upload, reads were mapped to the Plasmodium falciparum 3D7 v3.0 reference genome and only mapped reads were retained, to filter out human reads.
Supplementary materials relating to this paper can be found here: Figshare: An expanded method for malaria parasite genetic surveillance using targeted nanopore sequencing. https://doi.org/10.6084/m9.figshare.28539320.v1.27
The project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Thanks to Prof Olivo Miotto (MORU, Thailand), Dr Nhien Nguyen Thanh Thuy (OUCRU, Vietnam), Dr Keobouphaphone Chindavongsa (CMPE, Vientiane, Laos) and the GenRe-Mekong team for supporting use of P. vivax samples collected from Laos to test the 18S rRNA primers for Plasmodium species detection. Thank you to Dr Jacob Garcia and Dr Chiyun Lee (Genomic Surveillance Unit, Wellcome Sanger Institute, UK) for support examining primer sequences for variants in the MalariaGEN Pf7 data resource. Thanks to Dr Victor Asoala and the staff of Navrongo Health Research Centre research laboratories and the sample donors.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)