Keywords
CHAMPS, Stillbirth, Child Health, Mortality Surveillance, Autopsy, MITS, Laboratory, Sierra Leone
This article is included in the CHAMPS gateway.
More than four million child deaths occur annually; most are neither adequately documented nor investigated. The Child Health and Mortality Prevention Surveillance (CHAMPS) program was launched in Sierra Leone (SL) to generate high-quality data to determine definitive causes of stillbirths and under-five mortality (U5M) to inform decision-makers. Despite the multiple challenges of a greenfield research site, we highlight the experience of setting up a high-quality mortality surveillance (MS) system, including the viability of Minimal Invasive Tissue Sampling (MITS).
To establish the MS program, we implemented qualitative research and community entry, a sensitive system for timely identification and notification of deaths and followed CHAMPS standard operating procedures for investigating deaths and assigning accurate and definitive causes of death. CHAMPS in SL was implemented in four phases during 2017-2019 by a consortium. Enrolled stillbirths and U5M underwent verbal autopsy, clinical-data-abstractions, MITS, microbiology, molecular and histopathological diagnoses, and Determination of Cause of Death (DeCoDe).
CHAMPS achieved a 93% consent rate, capitalizing on existing Ministry of Health infrastructure, community involvement, and local ownership. As of December 2022, 3,433 deaths were registered, with 1,056 (31%) eligible for enrolment. Of 439 cases DeCoDed, 402 (92%) of case-families had received feedback on the cause of death. Using findings and recommendations from the DeCoDe experts, CHAMPS is implementing interventions to reduce stillbirths and U5M at CHAMPS SL, including clinical review meetings, provision of emergency drugs and routine child death audits.
Implementing innovative MS in a challenging context, such as SL, is possible. Building on local knowledge and infrastructure has enabled the CHAMPS project to achieve remarkably high consent rates, given the cultural, religious and sensitivity challenges surrounding seeking consent for MITS from caregivers who have just lost a child. The programme has invested significantly in upscaling local technical capacity for surveillance and laboratory diagnostics.
CHAMPS, Stillbirth, Child Health, Mortality Surveillance, Autopsy, MITS, Laboratory, Sierra Leone
Globally, child survival has witnessed remarkable progress over the past two decades. Under-five (U5) mortality decreased from an estimated rate of 93 deaths per 1000 live births in 1990 to 38 deaths per 1,000 live births in 20211. Despite progress at the global level, in 2015, approximately 5.9 million children died in sub-Saharan Africa (SSA) before their fifth birthday2, with a considerable proportion of deaths from preventable and treatable causes2,3. In most parts of SSA, mortality surveillance and autopsies are either non-existent or limited4,5, and policymakers rely on verbal autopsy or modeled data with built-in assumptions for cause of death determination6.
Over the last 30 years, Sierra Leone (SL) has achieved notable progress in reducing U5 mortality, from 260 deaths per 1000 live births in 1990 to 112 in 20197. Measures put in place to achieve this included the introduction of the Free Health Care Initiative8. However, the country's U5 mortality rate remains the highest in West Africa and fourth-highest globally9. SL is also known for being vulnerable to childhood endemic diseases like polio10 and measles11 and having a history of outbreaks such as the 2012 cholera outbreak12,13 and the 2014 Ebola outbreak14,15. These outbreaks revealed how difficult it was for the healthcare system to adequately respond to increased demand for health services, respond to outbreaks through advanced laboratory diagnosis and monitor diseases and events14,15. To understand all these challenges, a study conducted after the Ebola outbreak revealed gaps in mortality surveillance and the registration of vital events; this made a stronger case for the need to strengthen mortality surveillance capacity within SL16.
In 2017, in line with the Sustainable Development Goal (SDG) 3.2.1 (end preventable deaths of newborns and U5 by 2030)17 and to target public health action, the Government of SL worked with key local and international partners to establish a high-quality mortality surveillance system in SL’s Bombali District as part of the Child Health and Mortality Prevention Surveillance (CHAMPS) network. The CHAMPS program, supported by the Bill & Melinda Gates Foundation through Emory University, aims to generate accurate data on why children are dying to inform evidence-based strategies and policies that can prevent future deaths. As of 2022, the network comprised seven countries in SSA and South Asia and used a standardized autopsy approach involving minimally invasive tissue sampling (MITS) to ascertain causes of death. Findings at the CHAMPS Network18,19 have important implications for improving clinical practices and informing policy for public health priorities and programs in SL and possibly across West Africa and other countries in similar contexts.
This paper outlines the processes used to establish the CHAMPS site, site characteristics, lessons learned, challenges (technical, religious, cultural, resources, etc.), and opportunities for setting up a high-quality and innovative mortality surveillance system in a resource-constrained setting like SL. We also provide insights on successful approaches to navigating the complexities of implementing such a program through a consortium of partners.
Apart from its geographical diversity and having variations in risk for major diseases like malaria, HIV, and TB20,21, SL was selected as a CHAMPS site because it has one of the highest U5 mortality rates in the subregion9. With the two chiefdoms in Bombali as the catchment area, Bombali District has the second highest U5 mortality rate in the province after Port Loko District22. Bombali District also has a recurrent history of deadly disease outbreaks, including vaccine-preventable diseases and the 2014–2015 Ebola outbreak23,24. A 2015–2016 study that evaluated multiple death reporting streams with a focus on U5 and stillbirth revealed that stillbirths and deaths in U5 were underreported in the Bombali Sebora chiefdom16.
CHAMPS defined its catchment area (In Figure 1) using the chiefdom boundaries (Bombali Sebora and Bombali Siari) of the SL Population and Housing Geographic Information25. The CHAMPS SL catchment area has urban, peri-urban, and rural characteristics; population statistics were generated from the SL 2015 Census25,26. Additional health statistics variables were available from the SL Multiple Indicator Cluster Survey (MICS) report22, demographic health survey (DHS) results27, and other surveys from Statistics SL, Ministry of Health (MoH) and UNICEF7.
The SL's capacity for health research has grown over the years due to the 2014 Ebola outbreak, which exposed the weaknesses in the healthcare system28. Before the implementation of CHAMPS activities, postmortem specimen collection and laboratory capacity at the site were limited. Although clinicians receive training for lumbar puncture and other procedures at the only medical school in the country, these are rarely conducted at hospitals. The only pathology lab in the entire country was at the Connaught Hospital (a tertiary hospital) in Freetown. However, it was not in use until CHAMPS began its MITS collection and laboratory procedures in 2019.
Several laboratory-driven studies have been conducted over the past decade in SL, including an investigation during which the Bombali virus was discovered29, an Ebola vaccine trials through the STRIVE study30 and University of SL Ebovac study in Kambia31, and Lassa fever study32. In 2019, following the CHAMPS MITS launch, the Countrywide Mortality Surveillance for Action (COMSA)33 was implemented. COMSA has produced annual data on mortality and causes of death at national and subnational levels for use by the government and health policymakers. It uses the 2016 WHO verbal autopsy (VA) tool to track causes of death but does not conduct any diagnostics on postmortem biological samples33.
The CHAMPS site in SL is implemented by a consortium of non-governmental organizations (NGO), unlike other CHAMPS network sites implemented by research institutions or university teaching hospitals and training institutions (Table 1). Staff are hired locally and internationally by the implementing organizations, and they were trained by the CHAMPS Program Office (at Emory University) and Barcelona Institute for Global Health (ISGlobal) to adhere to CHAMPS protocols.
| Country | Sites | Implementing institutions | Year of Establishment |
|---|---|---|---|
| South Africa | Soweto | University of the Witwatersrand, Medical Research Council Vaccine and Infectious Diseases Analytical Research Unit (VIDARU), and National Institute for Communicable Diseases34. | 2015 |
| Ethiopia | Harar | Ministry of Health, Haramaya University, the London School of Hygiene & Tropical Medicine, and Haramaya Health Research (HHR) Partnership32,33. | 2015 |
| Kersa | |||
| Kenya | Kisumu | US Centers for Disease Control and Prevention Kenya Medical Research Institute (KEMRI) and Kenya Ministry of Health Henry M Jackson Foundation for the Advancement of Military Medicine35,36 | 2016 |
| Siaya | |||
| Mozambique | Manhiça district | Manhiça Health Research Centre (CISM), Eduardo Mondlane University of Medicine The Barcelona Institute for Global Health (ISGlobal), Mozambique Ministry of Health, and the country's National Institute of Public Health (INS)37,38. | 2016 |
| Mali | Bamako | University of Maryland's Centre for Vaccine Development (CVD) and Mali Ministry of Health39. | 2016 |
| Bangladesh | Baliakandi upazila | International Centre for Diarrhoeal Diseases Research, Bangladesh (icddr,b), Bangabandhu Sheikh Mujib Medical University (BSMMU) Institute of Epidemiology, Disease Control and Research (IEDCR), Johns Hopkins University40 | 2017 |
| Sierra Leone | Bombali | SL’s Ministry of Health (MoH), Crown Agents Limited, FOCUS1000, and World Hope International (WHI) | 2017 |
SL's Ministry of Health (MoH) provides supervisory oversight and a policy framework for CHAMPS SL. Between 2017 and 2018, the CDC provided technical oversight, eHealth Africa provided fiscal management, while ICAP provided technical leadership on laboratory, histopathology, data and informatics until 2022. Since 2018, Crown Agents, a not-for-profit international development company has progressively taken over eHealth Africa’s role (2018) and ICAP's technical roles (2022). Crown Agents is therefore the lead fiscal and technical management partner for the three implementing partners: FOCUS1000 remains the lead for socio-behavioral sciences (SBS) from the outset; while World Hope International (WHI) also remains responsible for all mortality surveillance (MS) activities from the outset.
SL is located on the western shoreline of Africa, bordering Guinea and Liberia. As of 2015, the country had a population of 7,092,11341 spread across 71,740 km2 (27,699 sq mi—Table 2). Although English is the official language, the most widely spoken languages are Krio, Themne, Mende, Limba, and Kono42,43. The majority of the population (78.6%) is Muslim, with Christians making up 20.8% and traditional religion less than 1% of the population42,44.
| Demographic Characteristics | Country-specific | District-specific | Site-specific |
|---|---|---|---|
| Sierra Leone | Bombali District | CHAMPS Catchment Area | |
| Population | 7,092,11341,45 | 606,54441,46 | 162,38341,45,47,48 |
| Population density per km2 | 103.949 | 99.9146 | 577.145,47,48 |
| Urban population % | 41%41 | 29%41 | 78%41 |
| Administrative divisions | 5 provinces41,49 16 districts 190 chiefdoms | 12 chiefdoms25,41,50 81 sections (Rural) 10 wards (Urban – Makeni city) | 2 chiefdoms41,45,47,48,50 4 sections (Rural) 8 wards (Urban – Makeni city) |
| GDP (current LCU) | 12.3 billion USD | NA | NA |
| Political stability index | -0.0351 | NA | NA |
| Male, adult literacy rate % (age >10) | 59.4%41 | 54.9%41 | Unknown |
| Female, adult literacy rate % (age >10) | 43.9%41 | 38%41 | Unknown |
| Birth rate | 35.4 per 1,00052 | Unknown | Unknown |
| Fertility rate, total (Births per woman) | 4.241 | 4.041 | Unknown |
| Infant mortality rate at time of site selection | 56 per 1,00022 | 68 per 1,00022 | Unknown |
| Neonatal Mortality rate at time of site selection | 20 per 1,000 live births22 | 31 per 1,000 live births22 | Unknown |
| Under-five mortality rate at time of site selection | 94 per 1,000 live births22 | 119 per 1,000 live births22 | Unknown |
| Maternal mortality rate at time of site selection | 1,165 deaths per 100,000 live births53 | Unknown | Unknown |
The CHAMPS SL site is located in Bombali District, comprising two chiefdoms—Bombali Sebora and Bombali Siari—which were initially one chiefdom before re-districting in 201725,42. The catchment area includes 96 communities, spread across an estimated area of 281 km², with a population of 162,38341 as of 2017. The site comprises parts of the district capital, Makeni City (population 125,970)41, with an estimated driving distance of 185 km from Freetown (Connaught Hospital), the country’s capital city (population density: 7,397/km²)54.
The site's healthcare services are delivered at three levels: (1) primary healthcare services at the community level are provided through peripheral health units (PHUs). PHU health services are provided by trained nurses, midwives, medical technicians called Community Health Officers (CHOs), etc.; (2) secondary health care services at the district level are provided through district hospitals, which act as referral hospitals for PHUs and provide more advanced healthcare services by nurses, midwives, lab technicians, CHOs, medical officers and medical specialists, etc. (3) Tertiary-level facilities are all located in the capital, Freetown55. The Makeni Regional Hospital, which hosts the mortuary and the main CHAMPS lab, is a district and regional referral hospital (secondary level)55. The site catchment area comprises 28 health facilities, including the Makeni Regional Hospital (MRH) five other hospitals and the associated catchment PHUs as shown in Figure 2.
Themne is the primary language spoken within the CHAMPS catchment area, followed by Krio, then Limba.
CHAMPS is a population-based surveillance study design that combines active and passive data collection methods from multiple sources to obtain comprehensive data on the causes of child deaths.
The inclusion (eligibility) criteria for enrolment in CHAMPS are residence in the catchment area within the last four months, confirmed stillbirth or U5 death (Non-MITS), and body available within 24 hours of death for MITS.
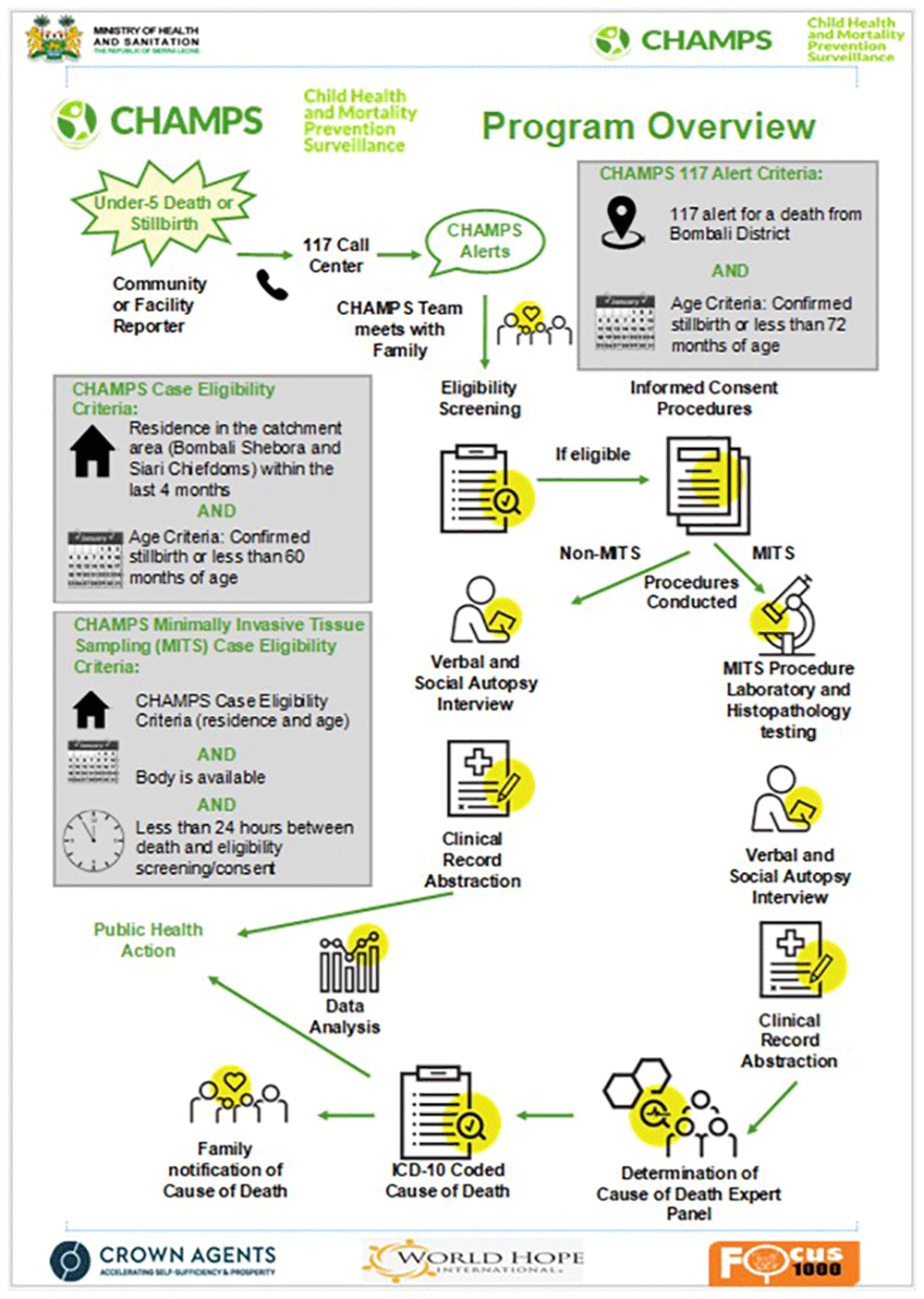
As previously described by CHAMPS` manual56, Salzberg, et al.47, and other CHAMPS Sites19,37, CHAMPS implementation occurs in a stepwise process (Figure 3). CHAMPS starts with community engagement to establish trust and ensure the acceptability and feasibility of CHAMPS activities. This is followed by mortality surveillance to identify, report, and investigate all U5 deaths and stillbirths. This process includes an eligibility assessment and informed consent process with the parents or guardian of the child. Upon consent, the body is taken to the mortuary for specimen collection using a minimally invasive tissue sampling (MITS) technique; MITS is a postmortem sample collection technique that uses biopsy needles to collect fluids and key organ samples56. These samples are taken to the lab for extensive testing. The surveillance team also visits family members and health facilities to conduct verbal autopsies using the WHO Verbal Autopsy tool57. The team also visits all health facilities the child or mother visited during illness and conducts a standardized clinical record abstraction of the child or mother. Once all the data and lab results are collated, an expert panel is convened and reviews all the information to determine the cause of death (DeCoDe). The DeCoDe findings are communicated to families, communities, and healthcare workers as feedback. Data may also be used to inform other interventions at all levels of the health care system or nationally through policy changes that can decrease U5 deaths and stillbirths. This process is defined as "data-to-action"56.
The CHAMPS SL site opted for a phased introduction of MITS to ensure sufficient time to engage with the community and address concerns adequately. The CHAMPS SL site adapted the standardized CHAMPS network protocol, procedures, and data collection tools described in Figure 358–60. This also involved developing a detailed CHAMPS SL protocol and Standard Operating Procedures (SOPs) and submitting them to the SL Institutional Review Board (IRB) for approval before commencing CHAMPS processes61,62. The following phases were deployed incrementally (Phase 1 to Phase 4) to build community confidence, establish local research capacity and build laboratory and surveillance infrastructure to absorb the entire CHAMPS methods from notification, recruitment, investigation and family/community follow-up processes.
In Phase 1, we employed a designed community entry strategy process that mapped out community structures and stakeholders, followed by courtesy visits to these identified stakeholders (Figure 4 and Figure 5). The SBS team, led by FOCUS1000, initiated CHAMPS community entry meetings in March 2017, where paramount chiefs, district stakeholders such as the Bombali District Chairman, Makeni City Council Mayor, and elected councilors were introduced to the program. This was followed by Participatory Inquiry into Community Knowledge of CHAMPS (PICK-CHAMPS)63 workshops for both leaders and community members to gauge their perceptions, cultural practices, and beliefs that may be impacted by CHAMPS methodology, especially the MITS sampling procedures (religious and cultural beliefs surrounding burial times and body handling prior to burial). After three months of PICK-CHAMPS, the findings enabled the CHAMPS team to adapt community engagement plans, consent processes, body handling, etc., to accommodate community perspectives. Similarly, these findings informed the subsequent adaptation of generic formative research activities for introducing CHAMPS to the broader community. The PICK-CHAMPS findings guided formative research on best practices for death notification and community engagement strategies, including community sensitization and participation. The team chose two peri-urban communities for the pilot phase to test the CHAMPS procedures. The SL site built on previous in-country research on the importance of community mobilization, engagement, and risk communication, such as the post-Ebola study, which emphasized the importance and benefits of consistent and ongoing mortality reporting15. To ensure a streamlined process leading up to MITS launch, the SBS team designed a series of activities to better understand community perceptions and provide ongoing engagements on CHAMPS activities:
Formative research was used to examine and assess critical aspects of the viability and perceptions of CHAMPS surveillance approach and factors that might hinder CHAMPS activities64,65. Emerging themes included 1) religious beliefs, 2) cultural norms, 3) political and economic conditions, 4) disease frequency and incidence, and 6) environmental factors. Formative research addressed the difficulties caused by the underlying community stigmatization surrounding deaths during the Ebola epidemic. Before any formative research study, participants provided verbal consent after the approved standard script was read to them. Semi-structured interviews, key informant interviews (KII) and in-depth interviews, and focus group discussions (FGD) were implemented for data collection and the results were shared with the wider implementation team to inform the next phase of CHAMPS activities and processes.
The CE team conducted weekly routine community meetings through the Community Liaison Officers (CLO) to cover all communities in each section within the catchment area66, with a target of visiting each community at least once a month. During these visits, CLOs addressed community concerns, looked out for rumors, addressed rumors if they arose, and gave community feedback regarding CHAMPS activities. The communities regularly invited CHAMPS staff during special occasions like celebrations, thanksgiving, sporting activities, or large community meetings and gatherings in their assigned communities, which they used as platforms to discuss CHAMPS activities and answer any questions. The collaboration between community members and the CE team during community events significantly contributed to the acceptance of CHAMPS research study and the MITS procedure.
The SBS team also partnered with the district-level MOH social mobilization pillar to promote maternal and child health activities in the CHAMPS catchment area. The partnership involved collaboration during community outreach events and health promotion campaigns, such as immunization, bed net distribution, nutrition drives, and health talks. These collaborations greatly improved the positive perception of the CHAMPS program.
The use of electronic media also helped increase acceptability, especially radio discussion programs led by trusted critical district stakeholders as panelists to address MITS concerns, promote child health, and give feedback. The radio discussions were routinely aired and simulcast across five radio stations in Makeni to reach people in the entire catchment area.
The SBS team trained religious leaders using scriptural references to promote maternal and child health messaging and promote acceptance, endorsement, and dissemination of CHAMPS messages.
To approach specialized communities and populations, such as military and police personnel, who often prohibit citizens from visiting their barracks until a permit is provided. The SBS adhered to their standard operating protocols, such as sending notice letters to heads to schedule meetings. The team organized separate engagement sessions for each of these specific groups and their dependents. These trainings and continuous engagement increased confidence between the soldiers and the SBS team, allowing unlimited access to the barracks for CHAMPS activities, including MITS. The team also attended muster parades to inform personnel who are regularly rotated or moved (to other duty station), as well as to provide CHAMPS information to newly transferred personnel.
The process involves collecting and analyzing unverified information or doubtful explanation that is circulating from various sources, including community members, health workers, and media reports about CHAMPS activities67. Each community engagement team member (CLO) conducted rumor surveillance in the section of the assigned catchment area. While doing routine community visits, a CLO, who also serves as a Rumor Surveillance Officer (RSO), gets information from Community Reporters (CRs) and other sources. CRs are approached and asked to share whatever information they have heard since their last visit. Whenever the rumor and its source were shared, the RSO followed up by talking to the individual or going to where the rumor originated to gain better understanding. Next, the RSO visited various locations in the community where people gathered, including "Ataya bases” (These are places where people gather to drink green tea and discuss burning community and national issues), "cookery baffers" (A traditional eatery or meal sale point) and the marketplace, among others. If any rumors were encountered, they were documented and addressed right away. CR and Facility Reporters (FRs) refresher training and stakeholder feedback workshops were also platforms that the team used to gather rumors. All the rumors were documented and shared with the core research team. High-priority rumors were immediately communicated to the site leadership, while other rumors were shared monthly with the team leads. Both the community engagement and core research teams prioritized the rumors-based on potential impact, and the research team created tools for investigation. Within 24 to 48 hours, the RSO and the corresponding research team member conducted a community follow-up on high-priority rumors. Low-priority rumors were addressed during the next routine visit. Examples of priority rumors from the community included the following: (1) the MITS process involved the draining all the blood from the body of a deceased child; (2) CHAMPS did MITS to remove body parts and organs from the dead children to sell for profit abroad; (3) community reporters were paid for every child that died and is reported; (4) CHAMPS was sent to the community to spread another dangerous disease, like Ebola, etc.
After seven months of understanding and engaging the communities, the site moved into Phase 2, where it introduced mortality surveillance (MS). Based on the formative research findings in Phase 1, the mortality surveillance processes were adapted with support from the MoH, including recruiting and training surveillance officers and community and facility reporters.
Phase 2 was divided into two parts. The first part (2A) piloted the identification and reporting of deaths in a few communities and facilities by community reporters and facility reporters (Figure 5), respectively, for five months. During Phase 2A, CHAMPS staff did not approach family members nor conduct mortality surveillance investigations. This phase was limited to three main hospitals in Makeni City and two peripheral health units in two peri-urban communities.
The notification process utilized the existing MoH reporting structures. During the Ebola response, a national call-in line, 117, was used for death notifications. CHAMPS leveraged this existing system for death reporting. Community and facility reporters were recruited and trained on the processes for identifying and reporting U5 deaths and stillbirths through 117 and the CHAMPS backup phone number (when 117 was unavailable). Periodic refresher trainings were conducted for the reporters.
In March 2018, the team transitioned to Phase 2B, where mortality surveillance investigations were conducted, including eligibility and consent, clinical abstraction, verbal autopsy etc. CHAMPS study staff did not collect postmortem samples during Phase 2B (so called “non-MITS”)56. Once the 117-call center received a death notification from the reporter for a potential CHAMPS case, a text message with identifiable information was automatically sent to the responsible CHAMPS study staff (Figure 5). Once the team received a death notification, they reviewed the information to determine eligibility56. For potentially eligible cases, a team was dispatched to conduct further investigations. CHAMPS surveillance officers (SOs) also used a smartphone app (Map.me68) to aid in the confirmation of whether the deceased’s residence was within the catchment boundary map. This process was performed in real time using an Android tablet.
All consent procedures were conducted in the parents' or guardians' local language, and copies of the paper consent forms were given to the family. This also included maternal consent for clinical abstraction (Figure 5). The site deployed all necessary steps to ensure the confidentiality and security of all participants' data.
The surveillance team also conducted active surveillance in all health facilities in the catchment area. It engaged health workers living in the communities to encourage them to report any community deaths. CHAMPS also leveraged monthly district health facility meetings to communicate the importance of death reporting to the nursing staff.
Below are the CHAMPS mortality surveillance investigations (Figure 5):
Mortality surveillance activities involved using standardized procedures and forms for clinical data abstraction. Following parental consent for MITS or Non-MITS, relevant clinical and demographic data were abstracted from maternal and child clinical records, if available at the facility. All data were entered electronically into REDCap. Data abstractors from the MS team visited all health facilities within or outside the CHAMPS catchment area where the deceased may have been managed during the illness to compile as much information as possible56.
This was a standard MS process that collected information about the circumstances that led to the child's death. CHAMPS surveillance officers used the 2016 WHO Child and Neonatal VA tool57, translated into two local languages, Themne and Krio. Unlike other sites, CHAMPS SL also collected social and demographic information from the deceased's household, using specially designed Social Autopsy (SA) questionnaires developed as part of an associated study on the social factors affecting stillbirths and U5 deaths69. As suggested by formative research findings, CHAMPS conducted all VA & SA interviews within 3–14 days from death notification.
In July 2018, nine months after the commencement of Phase 2 pilot, Phase 3 was started with a scale-up of the above surveillance investigations to the entire CHAMPS catchment area. This continued for eight months additional, leading up to the introduction of MITS and laboratory diagnostic activities in February 2019.
During Phase 3, CHAMPS established the MITS and laboratory structures in preparation for the launch of MITS specimen collection, laboratory testing, and histopathology. CHAMPS secured spaces at the MRH for MITS specimen collection, rehabilitated the MRH’s mortuary and designated a specific room for CHAMPS specimen collection.
As laboratory services and infrastructure were limited at the site, a new laboratory at MRH was constructed with the support of the CDC Foundation. Laboratory training and testing activities were initiated in late 2018 to early 2019 with the installation of advanced equipment and systems such as the Applied Biosystems™ QuantStudio™ 7 Flex Real-Time PCR System70 and TAC Nucleic Acid Extraction equipment for molecular testing, the BD BACTECTM™ FX40 Automated Blood Culture System71, the PHOENIX™ M50 Automated ID/AST System72 for microbiology testing, and GeneXpert XVI73 for HIV and TB testing. Laboratory staff were trained on the significant ancillary equipment required to process and test MITS samples56.
Since there was no functional laboratory for histopathology testing, CHAMPS equipped the histopathology lab at Connaught Hospital in Freetown, the capital of Sierra Leone. Barcelona Institute for Global Health (IS Global) deployed technical expertise to support the installation of the histopathology equipment and facilitated histopathology training on tissue processing before MITS activities could commence. In addition, three study personnel were sent to a 5-day MITS specimen collection training at the Hospital Central de Maputo, Mozambique, organized by the ISGlobal Institute of Barcelona. These MITS staff were equipped with the skills and knowledge required for an efficient MITS specimen collection procedure on their return to Sierra Leone.
In February 2019, the CHAMPS program transitioned to Phase 4, called the MITS phase. The MITS and laboratory (lab) structures were established during the MITS preparatory stage. On Feb 28, 2019, the first MITS procedure was performed at the site. This was a pivotal moment, following months of preparations.
As detailed in the CHAMPS Network manual56,74, three MITS staff members typically perform the MITS autopsy procedure (a MITS technician and two MITS assistants). This process includes preparation of the body (cleaning and sterilizing), gross examination (preparing the sample for diagnosis), anthropometric measurements (measuring the length of arm, head, leg, foot, etc.), photography, sample collection, and documenting MITS collection using the MITS collection forms. Samples such as cerebrospinal fluid, blood, nasopharyngeal secretions, liver tissue samples, lung samples, brain, placenta tissue, etc. are obtained56. Upon request, a family member may observe the MITS procedure.
Samples collected were transported to the MRH lab, where they are accessioned and distributed to the various labs for intensive testing. These include clinical, microbiological, and molecular testing at the MRH and histopathology testing at the Connaught Hospital Histopathology Lab in Freetown. Histopathological samples were also sent to the CDC Central Pathology Laboratory (CPL) in Atlanta, for advanced testing and quality control and quality assurance. Histopathology testing was performed at Connaught Lab and the CDC Lab on formalin-fixed, paraffin-embedded tissue specimens. Findings from both labs are discussed through telepathology sessions, during which findings and diagnoses are compared among site and CPL pathologists.
At the MRH, laboratory diagnosis was made for diseases such as malaria, HIV, TB, Sickle Cell Disease, etc., using SD Bioline (rapid test), a microscope, GeneXpert, etc. At the same time, microbiology (blood and cerebrospinal fluid culture) was done using BD Bactec, and molecular testing by TaqMan array cards (TAC)56 using the QS7 machine.
The CHAMPS SL DeCoDe panel was constituted and trained in October 2019. It consists of national and international medical experts, including a pediatrician, a neonatologist, a pathologist, an epidemiologist, a microbiologist, and an obstetrician. Local representation from the Bombali District, MRH hospital management, and clinicians who cared for the deceased were also present. All panelists underwent standard research, confidentiality, and COD determination training.
A DeCoDe case manager generates case packets from the CHAMPS database, which includes all data collected on case demographics, antemortem clinical summaries, VA, MITS data, and laboratory diagnostics. Each panel member reviews the packets before the meeting and assigns a preliminary cause of death (CoD) diagnosis, utilizing the CHAMPS Diagnostics Standards59 and the WHO Medical Certification of Cause of Death Guidelines (ICD-PM)75. These preliminary adjudications are discussed during the panel session to reach a consensus59. The expert panel also determines whether the death was preventable and makes recommendations to the MOH to inform needed interventions (‘Data-to-Action') to prevent similar deaths in the future. Some actions may be at an individual or family level, while others may be at a higher policy level. The final CoD and DeCoDe panel recommendations are summarized in preparation for family and community feedback.
The site introduced case mapping to nearby health facilities, as part of the SL site DeCoDe packets. This is a unique activity of the SL site in the CHAMPS network that provides the DeCoDe panelists with a concise summary of whether there were potential delays in reaching the health facility, when reviewed in conjunction with VA summaries. The data team develops distance maps from the deceased child's home to the nearest health facility and the main referral facility (MRH) using Quantum GIS – a geographic information system software. QGIS allows the team to analyze and edit spatial information with a standardized estimated time and distance to each health facility - walking and by motorbike. The travel times applied standard estimates of the distance and time of walking (12 to 14 minutes per kilometer) and using a motorcycle (20 to 30 kilometers per hour) to develop DeCoDe distance maps.
Feedback to the family may happen at various stages during the process. This may happen within a few days of death notification for notifiable infectious diseases, or within a few months for the final DeCoDe results. The MS surveillance team member(s) who conducted the verbal autopsy are responsible for returning results to the family. Periodically, an MS supervisor and an SBS team member may accompany the team in delivering the results. They provide the cause-of-death certificate that includes findings and recommended preventive measures to the family. The team also document the responses from family members upon communicating the DeCoDe result.
Data-to-Action, the last stage of the CHAMPS process, included using learnings from the findings in the preceding Phases or observations from DeCoDe findings to inform actions and policies to prevent future deaths (Figure 4 and Figure 5). Actions could be on an individual or family level or at a community or national level through policy changes. The national CHAMPS Advisory Committee and the local Data-to-Action Working Group were formed with MOH, MRH, and CHAMPS staff to examine findings and recommend immediate actions to reduce U5 deaths.
CHAMPS SL explored multiple opportunities to improve the uptake and use of CHAMPS data for policy and action at community, local and national levels. The CHAMPS National Advisory Committee and Local Data-to-Action Working Group were established in 2020 to spearhead these activities. The two groups reviewed and prioritized possible implementation ideas using the DeCoDe Panel recommendations for reducing stillbirths and U5 deaths. Examples of Data to Action activities, have included clinical review meetings with the scope of sharing findings of the CHAMPS DeCoDe panel with clinicians in the CHAMPS catchment. Often led by one of the DeCoDe panel experts, the clinical review meetings have been a critical forum to share findings and recommendations and jointly explore opportunities for improvement of clinical services – from the ground up. The local hospital and PHU clinicians are best placed to help identify additional gaps and use the CHAMPS findings to consider changes in practices or interventions that could prevent future under-five deaths and stillbirths. The Healthcare Workers Forum was an opportunity to publicly present the project's findings to clinical and public health students during their training at the local university. Child death audits at the MRH have become a weekly avenue to review two or three deaths and identify recurrent preventable causes of child death at the child emergency room and neonatal care units. These avenues have resulted in more open discussion of systemic and individual failures, with a focus on finding solutions rather than distributing blame. This has been a major cultural shift within the CHAMPS catchment area.
By design, the CHAMPS data is publicly and immediately available to researchers and the public at the network website76. CHAMPS data management system uses approved tools, systems, and secure servers to maintain data integrity and preserve patient confidentiality. The REDCap77 version 12 and SurveyCTO78 version 2 (Open Data Kit79 can also be used as an alternative to REDCap and SurveyCTO, since it is a free and open source tool) secure platforms are used to collect and manage the data quantitative and qualitative data respectively. Data are reviewed at multiple levels, and data quality issues are resolved using REDCap, Microsoft Excel, Stata, and other data systems. Deidentified data are further managed and backed up at the CHAMPS Program Office at Emory University in Atlanta in Lab-Key. Data outputs, including dashboards, monthly bulletins, presentations, newsletters, manuscript tables, etc., are regularly produced by the site for dissemination to key stakeholders across the country. GIS is also critical in the enrollment of cases during eligibility as well as for the DeCoDe process. GIS app, Map.me is a GIS tool installed on the tablets, along with catchment area maps, to assist surveillance officers during eligibility screening and catchment verification in enrolling eligible cases. QGIS is used to develop the DeCoDe map. Geocoordinates used are collected during the return of the body and VA procedures.
Before the initiation of CHAMPS SL in 2017, a comprehensive research protocol was submitted to the Sierra Leone Ethics and Scientific Review Committee (SLESRC) at the Directorate of Training and Research for Institutional Review Board (IRB) approval. The protocol included the CHAMPS's objectives, community engagement activities, surveillance processes, specimen collection, laboratory methods and procedures, DeCoDe processes, data collection tools, consent processes, and other required documents.
In adherence to the protocol, every CHAMPS staff member completed standardized research ethics training before starting work. Caregivers of potentially eligible participants were requested to provide verbal consent before screening for eligibility processes commenced. Furthermore, parents or guardians of deceased children were approached to provide written informed consent before the MITS and non-MITS procedures. All consent discussions took place in the parent or guardian's native language.
The CHAMPS research protocol was approved by the SLESRC on 3rd May, 2017, and subsequent CHAMPS annual progress reports are submitted to the SLESRC for annual ethics renewal. The most recent SLESRC approval number is 002/05/2024 with approved date 02/05/2024.
Following the implementing of a comprehensive approach and the standardized surveillance methods, the foundation resulted in an encouraging community and facility participation in the identification and reporting of deaths, Overall, CHAMPS-SL has received 3,433 unique death notifications, of which 1,056 (31%) were eligible for inclusion in the CHAMPS study (707 eligible for MITS and 349 for non-MITS; Table 3). Of the CHAMPS eligible, 986 (93%) consented to participate in CHAMPS processes, including 612 (87%) cases whose families consented for the full MITS procedure. A total of 604 (99%) MITS procedures were conducted among those eligible for MITS. As of December 2022, the expert DeCoDe panel had reviewed 439 of the 604 (73%) MITS cases. Of these 439, 402 (92%) of families had received feedback on the cause of death and how to prevent future deaths within the family and community.
CHAMPS SL has accumulated much learning throughout the project through formative research, rapid assessment studies, rumor surveillance, and community engagement activities. The team learned of socio-cultural, religious, and traditional norms, beliefs, and practices, which informed the feasibility and acceptability of CHAMPS activities. The CHAMPS consent rate (93%) was high, suggesting high community acceptance and testament to the early investments in community ownership efforts and a phased implementation approach.
First, we learned that it would be culturally unacceptable for CHAMPS to focus solely on investigating deaths without actively promoting child health and wellness. Similar to CHAMS Ethiopia`s learning19
Secondly, Rumors about CHAMPS trying to infect dead children with a new disease or attempting to cause a new outbreak of disease were rife. Communities spuriously associated CHAMPS mortality surveillance with the 2014 Ebola outbreak, which had significantly impacted our catchment area. Fresh memories of the 117 being used for notification of Ebola cases resulted in initial distrust for the CHAMPS death notification process, which utilized the same system. However, CHAMPS learned early that most communities had parallel informal death reporting mechanisms through community stakeholders such as chiefs and religious leaders. This created an opportunity to engage and collaborate with these established community structures.
We also learned that, regardless of religion, there was a cultural preference to bury dead children within one to four hours of death and in as little as 30 minutes for stillbirths. This posed challenges to gaining consent for CHAMPS, as the MITS procedures would prolong the time to burial by at least 2 to 3 hours.
Other learnings included how the body should be handled after death. While it was culturally required to give a token gift, generally a "Kasanke" (white) burial cloth, a few community members considered this a potential inducement to consent to the research procedures when visiting a bereaved family. As a result, CHAMPS opted to provide transportation of the body for burial to all families approached, regardless of whether they consented or not, because we didn't want people to know who did and didn't consent. This demonstrated a sign of respect and to show active empathy and support to grieving families. Similarly, dead bodies were expected to be buried in a wholesome manner with minimal scarring to the body. It was essential to emphasize that MITS was minimally invasive.
Finally, respondents said that their traditions should be respected when visiting their communities. And repeatedly emphasized the expectation that CHAMPS would utilize the findings to help implement local changes to reduce future child deaths in the communities.
The use of a phased implementation approach at the Sierra Leone site was essential to the success of the CHAMPS site. It allowed time to engage well with the community, reinforce surveillance systems, and build laboratory capacity and infrastructure and properly embed the study within the Ministry of Health as part of a holistic health system approach to find and resolve systemic issues. Further, the slower implementation allowed the team to integrate lessons learned at each stage of the scale-up.
CHAMPS’ commitment to sustainability by utilizing and building on existing infrastructure and capabilities, locally and nationally, was helpful for program implementation. This strategy took advantage of local structures, including local and national leadership at the community level and MoH. Additionally, CHAMPS leveraged systems implemented during the Ebola response, like the 117-call center, and improved the community perception of the call center. At the MoH level, CHAMPS worked closely with the District Medical Officer and other leaders of the District Health Management Team (DHMT), who usually oversee the overall management of health in Bombali District. The CHAMPS administrative office is currently co-located within the DHMT compound, and the MITS and laboratory facilities are located at the regional referral hospital in Bombali, i.e., the Makeni Regional Hospital. These co-location efforts facilitated and cultivated ongoing collaborative relationships with the DHMT’s leadership and social mobilization team, the district surveillance, and M&E teams, and the MRH mortuary and laboratory teams. At the national level, the Chief Medical Officer and the Deputy Minister of Health served and still serves as the Site Co-Director. This Co-Site Director was strategically involved in all strategic decision-making and directions for the project. This led to the participation of other directorates within the ministry and from different arms of the government.
Throughout its implementation, CHAMPS has capitalized on its resources to increase the ability of communities, health facilities, and staff to report deaths and conduct investigations, including enhancing laboratory capacity and establishing local cause of death certification in Sierra Leone. To help strengthen the district surveillance system, CHAMPS has trained over 500 community and facility reporters on timely death reporting procedures, reporting and investigation of notifiable diseases, and reporting and investigation of other unusual events. As an important side benefit, open avenues and for a to discuss health system challenges has provided community members a space and the boldness to jointly disclose and codesign strategies to improve their community health, with support from CHAMPS field staff.
Similar to other workstreams, the local laboratory team has undergone frequent refresher trainings with each update of the standard operating procedures and were kept up to date on the latest equipment, software, and procedures. The CHAMPS research laboratory now ranks is the most sophisticated in the country, with the laboratory staff providing training and support to other district and national laboratories as part of our corporate social responsibility. The new ultra-modern laboratory facility at the MRH (commissioned in July 2022) currently houses the CHAMPS and clinical labs, providing an opportunity to upgrade the laboratory capacity of the regional hospital and the clinical care of patients. This co-location has encouraged enhanced capacity building for district laboratory staff and university students, several of whom use the CHAMPS lab for their internships. Similarly, the recently revitalized histopathology lab at Connaught Hospital remains the only functional pathology laboratory in Sierra Leone. In addition to postmortem testing of CHAMPS MITS samples, the Connaught lab has enabled the MOH to reinstate diagnostic histopathological services, now serving as the sole hispathology laboratory for the entire country. Since 2022, clinicians across all 16 districts are able to send formalin-fixed samples to Connaught for testing. Prior to making this available, such samples were typically shipped abroad for testing (if patients could afford the costs); and due to shipping delays, histopathology results sometimes were returned after a patient had passed on or their cancer had metastasized.
Through its continuous surveillance capabilities, CHAMPS techniques and procedures provide evidence-based information for action at the local and national levels. The continued engagement of mortuary attendants at the MRH as part of the facility reporters has enhanced sensitivity of death reporting. To further support this effort, the hospital management implemented guidelines mandating all deaths to be conveyed to the mortuary in the first instance, even if relatives prefer immediate burial due to cultural norms. All mortuary workers were required to report all these deaths to CHAMPS for potential screening. In addition, the surveillance team collaborated with the SBS team to create radio messages and jingles about death reporting. These were constantly aired on the local radio stations at peak listening times, further encouraging community reporting of deaths.
All MITS cases are initially tested for notifiable diseases, such as HIV, TB, Ebola, COVID-19, and any other priority diseases, conditions, and events. All notifiable diseases identified are immediately reported through the CHAMPS leadership and surveillance teams to the appropriate MoH structures at the district and national levels (surveillance, M&E, HIV, TB teams, etc.,). This immediate reporting, prior to full testing of each case, has helped to facilitate an integrated response action between CHAMPS and MoH. CHAMPS often supports the MOH to conduct further investigation or contact tracing, as required by national protocols.
Integrating Geographic Information System (GIS) in CHAMPS surveillance and DeCoDe processes has become a critical component of our unique processes compared to the rest of the CHAMPS network. Use of Map.me for eligibility catchment verification and QGIS for quality management and maps were utilized to effectively reduce enrolling errors in the CHAMPS eligibility process, as they aided in confirming that all enrolled cases were within the catchment boundaries. During data analysis, this has also aided in geolocating cases based on residency, such as those from various chiefdoms, by determining whether they were urban or rural. The maps integrated into the DeCoDe packages have proven extremely valuable to the DeCoDe panels by providing the information to evaluate any delays or barriers that can hinder early reporting to health facilities. The display of the distance and time required to reach the nearest health facility and regional referral hospital (MRH) has aided in the accurate recommendations to the MOH and other partners in addressing the delays that may have led to the child's death.
The families of deceased children appreciated the one-to-one feedback sessions. These were private sessions held at their family homes, and provided opportunities to resolve misunderstandings, discussed how to improve the care of other children and sometimes advice for further testing, depending on the cause of death (e.g. for sickle cell disease, HIV, communicable diseases, etc.). At the hospital level, attendance of the management and staff at the CHAMPS DeCoDe panel meetings resulted in more in-depth discussions about mortality prevention and needed systemic changes to improve survival. As deaths were not systematically counted nor reviewed prior to CHAMPS, this has been one of the critical levers for change. The hospital matron's specific quick actions have included minor adaptations with a huge impact on neonatal and child survival, such as providing findings to the wards in charge and adapting the process for transferring neonates from the theater to the special care for baby unit (SCBU). The significant reduction in transfer times has guaranteed that those crucial minutes resulted in prompt interventions to reduce neonatal mortality.
The annual stakeholders’ summit, CHAMPS Advisory Forum, and feedback to the national and district stakeholders on CHAMPS activities have served as critical platforms to elevate CHAMPS findings to the appropriate levels, resulting in evidence-informed actions at various levels. These platforms have both enlightened and inspired representatives, including political leaders at the district and national levels, to take responsibility for preventing U5 deaths.
Dissemination of CHAMPS data to enhance public engagement or inform action, such as the clinical review meetings, healthcare worker forums, CHAMPS national stakeholder summaries, etc., were used as platforms to disseminate CHAMPS data and findings. Positive audience responses demonstrated the success of data sharing via presentations in forums, meetings, and conferences. A participatory community feedback method was established to improve participation, dramatically changing data sharing and consumption. This technique explored unique options, such as personal-interest stories, graphical representations of topics, radio discussions, and community drama plays, to vary the distribution approach and assure more extensive community participation.
Since December 2022, over 50 CHAMPS bulletins with the latest findings have been distributed to local and national leaders. In 2020, the Site designed a widely distributed online newsletter to periodically share its actions and activities with local, national, and international partners. All these CHAMPS information outlets have been used as references for evidence-based child mortality discussion at various MOH meetings and platforms, including monthly in-charge meetings, national directorate meetings and during donor discussions on future healthcare priorities.
At the national level, CHAMPS is a recognized member of key technical working groups and regularly share data with national managers and development partners. Examples include the reproductive and child health quarterly technical working group meetings and the health NGO network.
Data from the site has been used by CHAMPS and the MoH to achieve several quick wins by addressing immediate issues. CHAMPS SL's local Data-to-Action Committee has been more agile in addressing "low-hanging fruits," such as interventions that can be carried out locally with more focused effort and a significant impact. Such actions are implemented at multiple system levels—hospitals, communities, and districts.
Clinical review sessions for doctors and nurses as clinical feedback, facilitated by DeCoDe subject matter experts, have offered a chance for health workers to reflect on their actions throughout the management of the sick child or mother. It is used for continuous professional growth to improve clinical abilities and to remain updated on medical knowledge and standard operating procedures. It enables the identification of areas in patient care that require improvement. These meetings also improved communication and coordination by bridging communication gaps between peripheral health units and MRHs during referrals. They have been successful in supporting healthcare workers in understanding the importance of their commitments to these deaths and the impact that their level of effort will have. As a result, the MRH, notably the pediatric ward, recently commenced a quality assurance committee that meets regularly to review quality concerns affecting healthcare delivery at the hospital.
The MRH also benefited from CHAMPS efforts to upgrade the medical records management system and records storage facilities. The upgraded and furnished records room now provides better access to clinical records for both CHAMPS and clinical cases. Similarly, clinicians' documentation has anecdotally improved significantly since CHAMPS was launched, due to constant engagements, training, and regular review of patient folders for information. Other vital interventions included enhancing the daily briefing, updating, and ensuring adherence to policy guidelines and standard operating procedures, and improving infection prevention and control (IPC) at the wards.
During the COVID-19 pandemic, it was demonstrated how a well-funded mortality surveillance system, like CHAMPS, played a crucial role in Bombali District’s COVID-19 response. CHAMPS’s existing infrastructure provided a framework that was swiftly modified into a Bombali District preparedness and response strategy.
CHAMPS' strengths were evident across multiple response pillars: the CHAMPS SBS team was leading the mobilization and engagement on the dos and don'ts of preventing COVID-19; support to the district leadership for coordination of response activities; disease surveillance, with CHAMPS surveillance officers spearheading efforts to identify, notify and investigate all COVID-19 alerts; and logistical support through use of CHAMPS vehicles, bikes, and a conference hall for daily surveillance response meetings.
With its infrastructure and well-trained technical personnel on site, CHAMPS SL is exploring additional research opportunities to leverage these investments and advance goals linked to supporting children and better calibrating mortality rates. In 2020, CHAMPS received a supplement to collaborate with the University of Toronto to conduct minimally invasive tissue sampling (MITS) on 200 U5 deaths for the Countrywide Mortality Surveillance for Action (COMSA) project in Bo District. This project has now completed enrolment (as of February 2022) and in the process of using MITS DeCoDe information to calibrate and validate verbal autopsy information obtained by the COMSA project in their national surveillance effort. Secondly, CHAMPS is collaborating with WHO and the Fleming Fund project on enhancing clinical antimicrobial resistance testing at the MRH. The goal is to improve clinical sample testing from U5 children so as to better inform antimicrobial prescription and stewardship by local clinicians. Thirdly, the Sierra Leone site has commenced the first health and demographic surveillance system in Sierra Leone. Baseline enumeration of the entire catchment area was concluded; this will provide more credible denominators for calculate rates and act as a platform for future studies. Fourthly, the site has established pregnancy surveillance to fully monitor pregnancy outcomes prior to 20 weeks of gestation. It is hoped that this will improve early linkage to antenatal care and reduce perinatal mortality.
Countries with the highest child mortality are often similar to Sierra Leone – with limited local research capacity, limited laboratory infrastructure, cultural and religious beliefs that may limit the acceptability of postmortem sampling, historical contexts that may limit trust in the government and in external research initiatives, limited access to institutional establishments, limited pool of trained scientists and poor access to the infrastructure and utilities to establish and sustainability run an advanced research study. Yet, we found that an advanced mortality surveillance program could be implemented in a low-resource setting like Sierra Leone using systematic and standardized processes, early community involvement, and MoH support. Implementation of CHAMPS has increased human resource capacity and improved research infrastructure in our study catchment area and the capital city. These new resources should now be further leveraged to support routine clinical care for Sierra Leoneans, improve the training available in local universities and support response activities in a future outbreak or pandemic.
The cornerstones of the strategy for reducing child mortality will continue to include translating CHAMPS data into action, communicating relevant findings to appropriate audiences, and enhancing collaboration between MOH and other partners, with the aim to encourage policy changes and consistent adherence to established protocols. CHAMPS has become well-established and accepted within the catchment area and Sierra Leone at large. In the coming years, CHAMPS will leverage its relationships and research infrastructure to continue to reduce child mortality, while supporting ongoing upgrades to health service delivery and community acceptance of health interventions in general.
Before the initiation of CHAMPS SL in 2017, a comprehensive research protocol was submitted to the Sierra Leone Ethics and Scientific Review Committee (SLESRC), Directorate of Training and Research for Institutional Review Board (IRB) approval. The protocol included the CHAMPS's objectives, community engagement activities, surveillance processes, specimen collection, laboratory methods and procedures, DeCoDe processes, data collection tools, consent processes, and other required documents.
The protocol also stipulated that every CHAMPS staff member must complete standardized research ethics training before starting work. Caregivers of potentially eligible participants were requested to provide verbal consent before screening for eligibility. Furthermore, parents or guardians of deceased children were approached to provide written informed consent before the MITS and non-MITS procedures. All consent discussions took place in the parent or guardian's native language.
The research protocol was approved by the SLESRC in 2017, and subsequent CHAMPS annual progress reports are submitted to the committee for annual ethics renewal. The most recent SLESRC approval number is 002/05/2024 with approved date 02/05/2024.
CHAMPS_December_2022_Bulletin in PDF Format: The CHAMPS monthly bulletin "CHAMPS SL December 2022 Data bulletin” provides comprehensive details and data on CHAMPS data collected.
There are other related CHAMPS studies with data listed via CHAMPS Dataverse https://dataverse.unc.edu/dataverse/champs.
We would like to thank the community members who have supported and/or taken part in the research to date, including those who have lost children and all our local stakeholders. We express our gratitude to the Ministry of Health for supervising the implementation from the outset. We also thank the Sierra Leone ethics committee for the oversight and guidance, and all founding partners for technical support and guidance. For a successful implementation, we also acknowledge and thank the research staff, implementing partners, and the CHAMPS Program Office at Emory University.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Yes
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: health policy and systems, maternal and newborn health, maternal death surveillance and response
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Yes
Are sufficient details provided to allow replication of the method development and its use by others?
Yes
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 19 Sep 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)