Keywords
anaerobic digestion, Ascaris suum, electrochemical cell, faecal sludge, helminth
anaerobic digestion, Ascaris suum, electrochemical cell, faecal sludge, helminth
Faecal sludge and wastewater reuse for agriculture, land irrigation, and groundwater recharge are becoming standard practices worldwide. When dealing with treated faecal sludge or wastewater the most crucial parameter to ensure safety is the pathogen content.
Coliform is the main bacterial pathogen routinely monitored in wastewater treatment plants. Another important group of pathogens monitored regularly are parasites (Helminths). Helminthiasis is a neglected tropical disease caused by helminths (Ascaris lumbricoides (roundworm), Necator americanus (hookworm), Ancylostoma duodenale, and Trichuris trichiura (whipworm)) along with others. These worms are responsible for malnutrition, anemia, and impaired cognitive development in humans (WHO, 2012; WHO, 2015). Helminths have been monitored since WHO published guidelines to control the number of helminth eggs in 2006. It is advised to limit the content to one helminth egg per litre of water (1 HE/L) and one egg per gram of total dry solids (1 HE/g TS) for sludge when applying wastewater, excreta, or sludge to crops for raw consumption (WHO, 2006). When comparing different types of helminths and the percentage infecting humans, Ascaris lumbricoides is the most commonly found helminth (Jimenez et al., 2002). Most studies have used Ascaris suum (which can infect pigs) as the indicator helminth to check the efficiency of wastewater treatment processes as it resembles the most resistant infectious helminth in humans, Ascaris lumbricoides (Kato et al., 2003; Manser et al., 2015; Popat et al., 2010).
Some technologies are available to inactivate helminth eggs. However, there is still a considerable gap, leading to untreated water being discharged into river bodies. The methods for inactivating helminth eggs currently being used are either costly or subpar. Technology advancement is required to ensure the complete inactivation of helminth eggs and to make treatment methods sustainable and affordable.
Anaerobic digestion (AD) is one of the most comprehensive technologies used for the treatment of helminth eggs (Machnicka & Grübel, 2022; Olsen & Nansen, 1987; Ruiz-Espinoza et al., 2012). Despite being the most used technology, AD's effectiveness against helminth eggs has not been extensively studied. Anaerobic conditions, temperature, pH, and intermediate products (volatile fatty acids, ammonia), which form during anaerobic digestion, help in the inactivation of pathogens including helminths (Fidjeland et al., 2015; Pecson & Nelson, 2005; Rojas-Oropeza et al., 2017). Due to insufficient biogas production from the anaerobic digestion of faecal sludge alone, co-digestion of faecal sludge with food waste was carried out.
Electrochemical techniques are also being used to treat pathogens in wastewater efficiently (Fores et al., 2023; Lin et al., 2020). When anode and cathode electrodes are subjected to a source of current, free radicals (hydroxyl radicals) are produced. These radicals are not specific to recalcitrant organic compounds and cell components, causing pathogen inactivation. The electrochemical process can result in the production of hypochlorite and the change in the pH of the solution together leads to the inactivation of helminth eggs (Talekar et al., 2018).
This study aimed to understand the role of anaerobic digestion and electrochemical process in helminth egg (A. suum) inactivation.
Experimental setup. The biochemical methane potential (BMP) assay was performed in serum bottles (130 mL) to co-digest faecal sludge (Obtained after solid-liquid separation of septic tank sewage from the sewage treatment plant at Baina, Goa) and food waste (Collected from one of the messes of BITS Goa). The ratio of food waste to sludge in the bottles was 5:1. Before seeding the serum container, the sludge's total solid (TS) and volatile solid (VS) contents were evaluated. Each bottle was subjected to a loading rate of biomass of 1.5kg VS/m3; the amount of faecal sludge and food waste to be added to the bottles to achieve desired loading rate was calculated using the VS values. VS was first calculated in kg/m3 for a 5:1 ratio and then converted to grams/100mL as the final bottle volume was 100mL. Macro and micronutrients, sodium bicarbonate, digested from a large-scale anaerobic reactor (Plug flow reactor of 60 m3 capacity constructed at BITS Goa for the treatment of food waste to produce biogas) as inoculum were added, and the final volume was raised to 100 mL by adding distilled water. A. suum eggs were procured from Excelsior Sentinel, USA, a mixture of viable and non-viable eggs (Figure 1). Eggs of concentration 8 eggs/mL with 63% viability were spiked in the BMP bottles. The experiment was performed using two bottles in each set; one bottle from each set was taken out at each sampling point (Day 0, 3, 6, 9, 15, 20, 25, 30, 35, 40, and 45) for analysis, and another bottle along with the remaining bottles from the set was used to take the gas reading every day. When the bottle was taken out at each sampling point, a gas reading from one bottle was considered a duplicate reading to perform analysis. The setup was maintained at room temperature (28 ± 2°C).
Parameter analysis. The different experimental parameters analyzed are given in Table 1. Biogas production was estimated daily by measuring the gas volume generated using a water displacement unit. A water displacement unit was made in the laboratory: a 5000 mL glass beaker was taken, and a 100 mL measuring cylinder was attached inside in an inverted position. The beaker was filled with distilled water until the zero mL mark on the cylinder, and gas from the bottle was passed inside the cylinder using a syringe with an IV tubing set. As the gas passed into the cylinder, it displaced the water, and the reading was recorded in mL. pH, TS, and VS analyses were conducted at each sampling point. PH was checked using an Oakton pH meter. The pH probe was dipped into the sample and the reading was taken. Readings were taken in duplicate. To plot a graph, standard deviation was calculated using the duplicate pH reading. For TS and VS analysis, the weight of the empty crucible was recorded, then the sample was added and the total weight was recorded. Sample weight was calculated by subtracting the crucible weight from the total weight (For the solid sample around 5 g of sample and for the liquid sample around 10 mL of sample was taken). Crucibles containing samples were kept at 105°C for 12 hours and the weight was recorded. Then the crucibles were ignited in a muffle furnace at 550°C for four hours and weighed once they had cooled down. TS was calculated by subtracting the crucible weight after the 105°C readings. VS was calculated by subtracting the 550°C weight values from the 105°C weights. Both TS and VS was then converted to per gram and then to percentage. To plot a graph, standard deviation was calculated using percentage values of both TS and VS.
| Parameters | Method/Instrument used | |
|---|---|---|
| 1. | Biogas | Water displacement unit |
| 2. | pH | Oakton pH meter |
| 3. | Total solids (TS) | Apha (2012) |
| 4. | Volatile solids (VS) | Apha (2012) |
| 5. | Volatile fatty acids (VFA) | Apha (1998) |
| 6. | Biogas composition (CH4, CO2, H2S) | Gas chromatography (Trace 1110_Thermofisher Scientific) |
| 7. | A. suum inactivation | Pebsworth et al. (2012) |
Biogas composition (CH4, CO2, and H2S) was analyzed using gas chromatography (GC). The GC instrument (Trace 1110, Thermofisher Scientific) was equipped with a packed stainless-steel column of spherocarb support, a thermal conductivity detector (TCD), and hydrogen as the carrier gas. The injector temperature was set to 150°C and the detector temperature to 185°C. Gas sample was taken from BMP serum bottles using a 2 mL syringe and injected into the GC instrument. Gas composition was determined by following the steps in the software (Chrom-Card data system, Version 2.12, August 2014) present in the instrument itself.
Volatile fatty acid (VFA) analysis was conducted using the titration method. A 5 mL sample was taken from the opened serum bottle, and the volume was raised to 100 mL using distilled water. The diluted sample was titrated in a glass beaker using 0.1 N HCl until the pH reached 3, and the burette reading (A) was recorded. Then 0.1 N NaOH was added until the pH reached 6.5, and the burette reading (B) was recorded. The following formula was used to calculate VFA (mg/L)
For helminth eggs analysis, the sample was taken in a plastic beaker and ammonium bicarbonate solution was added; the sample was mixed on a magnetic stirrer (Cole-Parmer- Stuart-UC152D) for 20 min. The resultant solution was passed through a 100-micron sieve (Analysensieb test sieve, 200 mm diameter, Fritsch, Germany) kept on top of a 20-micron sieve. The sample was washed with tap water, and the contents of the 20-micron sieve were placed in four 15 mL centrifuge tubes and centrifugated at 3000 rpm in a swing out rotor centrifuge (Eltek, MP 800) for 10 min. After centrifugation, the supernatant was discarded and zinc sulphate solution of 1.3 specific gravity was added to the pellet to the 14 mL mark with 3 ml solution added at a time and mixed on a vortex (Neuation, Digital Vortex Mixer iSwix VT) and centrifuged again at 2000 rpm for 10 min. The supernatant was carefully placed in a small 20-micron sieve (100 mm diameter) and washed thoroughly. The contents of the sieve were transferred to a centrifuge tube, and centrifugated at 3000 rpm for 10 min. The pellet was made into suspension by adding distilled water (1–2 mL) for microscopy using distilled water (UKZN PRG helminth method (Pebsworth et al., 2012). Using the ZEISS Primo Star microscope, eggs were observed and counted under 10X and 40X magnification. Images were captured by using iDS uEye Cockpit software (Version 4.96.1) which comes with the microscope. All parameters were examined in duplicate every third day for the first 15 days, then every fifth day until day 45. All the methods in detail are uploaded on Dryad (Mutnuri & Patil, 2023).
Statistical analysis. All the data were checked for normality and homogeneity of variance. The relationship between A. suum inactivation and all the other parameters was studied using correlation analysis. Statistical tests were performed using SPSS (IMB SPSS Statistics 25) software.
Experimental setup and experiment. The laboratory-scale electrochemical cell (EC) was set up in a 1000 mL beaker (Figure 2). A titanium plate was used as an anode electrode, coated with 6 µm thick mixed metal oxide (Ruthenium, Iridium, and Titanium in 70%, 20%, and 10%, respectively). The cathode electrode was made of SS 304 stainless-steel mesh. A variable DC supply unit (Laboratory DC power supply, Gwinstek, GPS-4303) was used to apply a consistent voltage. The electrochemical cell solution was continuously mixed with a magnetic stirrer (SPINIT Motorless Magnetic Stirrer, Tarsons) to generate hypochlorite, which was then employed as a disinfectant for inactivating A. suum eggs.
Hypochlorite generation using NaCl has been done with different electrochemical processes (Asokan & Subramanian, 2009; Gogoi et al., 2022; Spasojevic et al., 2015). This study generated hypochlorite as described in Gogoi et al., 2022 with a few modifications. Electrolysis of 2% NaCl solution was performed for two hours, producing 4700 ppm hypochlorite. The generated hypochlorite was diluted in various ratios with distilled water (1:5, 1:10, 1:20, 1:30, and hypochlorite without dilution). Helminth eggs (8 eggs/mL; 86% viability) were spiked into all diluted solutions, and helminth inactivation was observed under a microscope at 15 min, 30 min, hourly intervals between one and six hours, and 24 hours.
Figure 3 (a) depicts the biogas produced in the BMP assay. On the first day, the gas produced was between 112 and 122 mL, and on day 30, the overall gas generation (cumulative) was 1218 to 1389 mL. After 25 days, gas production began to decline; thus, recording readings were discontinued at 30 days. The produced gas was then examined using GC.
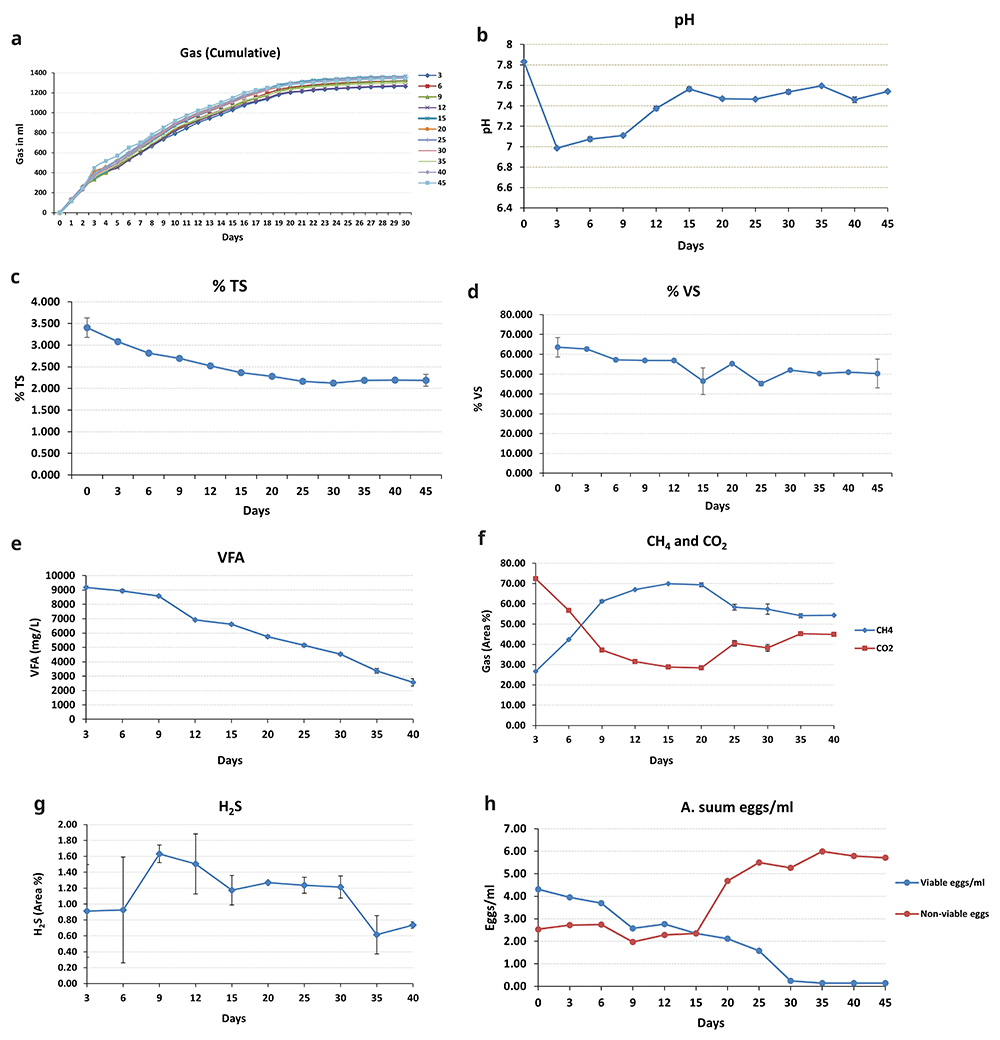
a) Cumulative biogas production, variations in the parameters b) pH, c) TS, d) VS, e) VFA, f) CH4, CO2, g) H2S, h) A. suum inactivation eggs/mL.
Throughout the experiment, the pH of the biomass remained approximately neutral (7–8) (Figure 3(b)). It was 7.83 at the start of the experiment (day 0), but it dropped to 6.98 at the next sampling point (day 3). It then went up to 7.37 and remained between 7.3 and 7.5 for the remainder of the incubation period.
The amount of TS and VS in the biomass reduced as the incubation progressed (Figure 3(c) and (d)). On day 0, TS was 3.40%; by day 45, it had dropped to 2.18%. VS followed a similar pattern, peaking at 63.53% on day 0 and dropping to 50.33% on the 45th day of incubation.
The VFA production in the biomass was highest on day 3 (9181 mg/L), which then decreased as the incubation period proceeded (Figure 3 (e)), dropping down to 2572 mg/L, observed on the last sampling day.
The trends for CH4 and CO2 were diametrically opposed (Figure 3 (f)). CH4 climbed to 69% until the 20th day, then declined to 54.33% until stabilizing on the 40th day. CO2 levels were high at 72.38% at the first sampling point (day 3). It declined (28.38%) until the 20th day of incubation and then increased slightly until the experiment ended. Throughout the trial, H2S displayed an uneven trend (Figure 3 (g)). The maximum H2S concentration (1.63%) was found on day 9, while the lowest (0.61%) was observed on day 35.
The total number of viable A. suum eggs dropped over the course of the experiment, contributing to the increase in non-viable eggs (Figure 3 (h)). The initial viable egg count was roughly 4 eggs/mL, reducing to 0.15 eggs/mL on the 35th day (which meets the discharge standard according to WHO, 2006). Inactivation of eggs was relatively low for the first 15 days (2.35 eggs/mL) and then continued to increase, with 98% inactivation achieved by the 35th day of incubation. There was not much additional variation in the number of viable eggs after day 35 (0.14 eggs on the 40th day); inactivation remained at 98%, so the experiment was stopped at 45 days. After 35 days, the number of non-viable eggs dropped marginally; this could be attributable to the complete disintegration of a few dead eggs that were not counted. Figures 4 (a–f) show representative pictures of A. suum on day 45.
Since the data for a few testing parameters was not normally distributed, Spearman's correlation analysis was used to individually analyze the relationship between the number of non-viable eggs and each test parameter. The analysis results demonstrated a positive association between pH and non-viable eggs (rs = 0.465, p = 0.022), indicating that the number of non-viable cells will also increase as the pH rises. TS and VS had a substantial negative connection with non-viable eggs (rs = -0.752, -0.607, and p = 0.000, 0.002, respectively). VFA also had a strong negative correlation with non-viable eggs (rs = -0.840, p = 0.000), indicating that increasing VFA concentration alone will not contribute to the inactivation of helminth eggs. The CH4 and CO2 values were not statistically significant (p = 0.474, 0.563, respectively), indicating that the effect is insignificant in this case. H2S was negatively correlated with non-viable eggs (r = -0.555, p = 0.011).
None of the different hypochlorite concentrations demonstrated evidence of helminth eggs being inactivated until six hours into incubation in the diluted hypochlorite solution. Only 10% inactivation was achieved in the 1:5 ratio (940 ppm) solution after a 24-hour incubation period. When exposed to absolute hypochlorite (without dilution), some of the A. suum eggs were quickly inactivated and over 24 hours, their inactivation was maximized. Table 2 shows the parameter values, and Figure 5 shows A. suum egg images before and after inactivation in hypochlorite solution.
The helminth inactivation study was conducted at a selected food waste and faecal sludge ratio. The ratio was selected as it demonstrated optimal biogas production in Shet and Mutnuri (2021). The BMP assay achieved 98% inactivation of A. suum eggs at the lab scale.
Pathogen inactivation in AD is as complex as the biogas generation process. Many parameters influence biogas production. In the current investigation, sodium bicarbonate was added to the serum bottles to limit the pH from becoming extremely acidic or basic (the pH stayed between 7–8), as doing so might hinder methane generation. Although the results did not directly indicate a relationship between pH and helminth inactivation, Spearman's correlation analysis revealed a positive correlation between pH and the number of non-viable eggs. According to correlation analysis, a basic pH is necessary for helminth inactivation, which aligns with what Mignotte-Cadiergues et al. (2001) observed. According to Pecson & Nelson (2005) and Fidjeland et al. (2015), In the absence of ammonia, basic pH (9–12) has no direct effect on the inactivation of Ascaris spp. Free ammonia (NH3) is considered more dangerous to pathogens because of its lipophilic nature, allowing it to penetrate through cell membranes without restriction, dissociate inside cells to shift ∆P across the cell membrane and generate an alkaline pH (Pecson & Nelson, 2005). pH, in combination with high temperature, was also found to be effective in helminth egg inactivation (Pecson et al., 2007).
TS and VS do not directly impact helminth inactivation but are essential to track biogas generation. These parameters showed a strong negative correlation with non-viable A.suum count; their value decreased as the non-viable eggs increased.
Numerous studies have reported that VFA production helps in the inactivation of helminth eggs (Puchajda et al., 2006; Rojas-Oropeza et al., 2017). It’s also reported that the free VFAs are more effective in helminth inactivation than ionized ones as they are lipophilic and membrane permeable (Puchajda et al., 2006). The equilibrium of free and ionized VFA depends on the AD's pH and temperature (Kunte et al., 2000). According to Riungu et al. (2018), the concentration of free VFA required to inactivate helminth eggs ranges from 4800 to 6000 mg/L. Despite reaching a certain concentration, VFA cannot inactivate eggs unless the pH is acidic (Harroff et al., 2017). The maximum VFA concentration in this experiment achieved was 9181 mg/L, which is adequate to inactivate helminth eggs; perhaps because sodium bicarbonate was added to the serum bottle, the pH didn't change or turn acidic, and there was no positive effect shown in the data. Additionally, the biogas (CH4, CO2, and H2S) does not directly influence the inactivation of A. suum; this was validated by correlation analysis because the data were not statistically significant except for H2S.
A. suum inactivation in the electrochemical process was achieved at the highest (4700 mg/L) concentration of hypochlorite. The experimental results demonstrated that inactivating eggs with a lower hypochlorite concentration (Table 2) was not highly effective. The results by Talekar et al., 2018 also indicated that chlorine concentration alone would not enhance inactivation. The inactivation of helminth eggs is influenced by a number of parameters, including the interaction of pH and free chlorine concentrations. Depending on the pH of the solution, hypochlorite persists in two forms (HOCl and OCl-). The percentage of HOCl decreases, and the percentage of OCl- increases as the pH of the solution increases. HOCl is approximately eighty times more potent than OCl- because uncharged HOCl is more effective in penetrating cell walls. Additionally, compared to other chlorine-based disinfectants, it responds more quickly to oxidation processes involving the organic matter or the essential elements of microbial cells. In the current experiment, the highly concentrated hypochlorite was diluted in various ratios, producing a more basic final solution that reduced the inactivation rate of A. suum eggs.
In the present study, anaerobic digestion was successful in A. suum inactivation compared to the electrochemical process. Multiple factors influence inactivation. The following are the critical conclusion points from the present study.
1. Anaerobic digestion is helpful in the inactivation of helminth (A. suum) eggs.
2. The inactivation of A. suum eggs by anaerobic digestion is influenced by a combined effect of more than one factor, which needs to be studied in detail, and also with the high initial concentration of A. suum eggs.
3. Hypochlorite concentration or pH alone can’t deactivate helminth (A. suum) eggs in the electrochemical treatment.
4. Factors affecting inactivation need to be studied in detail to achieve complete inactivation of A. suum eggs.
Dryad: Underlying data for ‘Study of helminth eggs (Ascaris suum) inactivation by anaerobic digestion and electrochemical treatment’, https://doi.org/10.5061/dryad.rbnzs7hg4 (Mutnuri & Patil, 2023)
This project contains the following underlying data:
1. A.suum_statistical_data__file_PP_and_SM_CSV.csv
2. A._suum_PP_and_SM_CSV.csv
3. Biogas_composition_(CH4__CO2_and_H2S)_PP_and_SM_CSV.csv
4. Biogas_PP_and_SM_CSV.csv
5. pH-PP_and_SM_CSV.csv
6. Total_Solids_(TS)_and_Volatile_Solids_(VS)_PP_and_SM_CSV.csv
7. Volatile_Fatty_Acids_(VFA)_PP_and_SM_CSV.csv
8. README.md
9. README.txt_File_PP_and_SM.txt
10. Supporting_figures_PP_and_SM.pdf
11. A._suum_stat_data_output_file_PP_and_SM.spv
12. Supporting_tables_PP_and_SM.pdf
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors greatly acknowledge DBT-BIRAC and Bill & Melinda Gates Foundation for supporting and funding this research through the "Empowered Septic Tank as decentralized wastewater treatment system" project under Phase II of the "Reinvent the Toilet Challenge."
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Water and waste water management, Circular economy, Solid waste management, Nanomaterials for Environmental applications
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Anaerobic digestion, waste activated sludge, power-to-gas
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 12 Feb 24 |
read | read | |
|
Version 1 12 Jun 23 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)