Keywords
mosquito, dengue, model, arbovirus, policy, intervention, Wolbachia, cost
The global prevalence of diseases transmitted by Aedes aegypti mosquitoes, such as dengue, Zika and Yellow Fever, is increasing, but development of promising new mosquito control technologies could reverse this trend. Target Product Profiles (TPPs) and Preferred Product Characteristics (PPCs) documents issued by the World Health Organization can guide the research and development pathways of new products and product combinations transitioning from proof of concept to operational use.
We used high resolution global maps of the case and economic burden of dengue to derive programmatic cost targets to support a TPP for Wolbachia replacement. A compartmental entomological model was used to explore how release size, spacing and timing affect replacement speed and acceptability. To support a PPC for a hybrid suppress-then-replace approach we tested whether Wolbachia replacement could be achieved faster, more acceptably or at a lower cost if preceded by a mosquito suppression programme.
We show how models can reveal trade-offs, identify quantitative thresholds and prioritise areas and intervention strategies for further development. We estimate that for Wolbachia replacement to be deployable in enough areas to make major contributions to reducing global dengue burden by 25% (in line with 2030 WHO targets), it must have the potential for cost to be reduced to between $7.63 and $0.24 (USD) per person protected or less. Suppression can reduce the number of Wolbachia mosquitoes necessary to achieve replacement fixation by up to 80%. A hybrid approach can also achieve fixation faster and potentially improve acceptability, but may not justify their cost if they require major new investments in suppression technologies.
Here we demonstrate the value dedicated modelling can provide for interdisciplinary groups of experts when developing TPPs and PPCs. These models could be used by product developers to prioritise and shape development decisions for new Wolbachia replacement products.
mosquito, dengue, model, arbovirus, policy, intervention, Wolbachia, cost
The first version of this manuscript has been updated in response to the reviewers’ comments.
For the entomological model, the text has been updated to clarify that the model was calibrated using data from the Yogyakarta trial and therefore best represents egg releases rather than adult mosquito releases. The suppression literature-derived efficacy values for male Wolbachia release have been updated and values for Sterile Insect Technique newly introduced, with subsequent updates to Figures 4, 5, and 6, Supplementary Figure 2, and Supplementary Table 1, in addition to minor text edits. Furthermore, the text has been updated throughout to highlight that suppress-then-replace scenarios are likely to be most relevant in circumstances where suppression is already part of an existing vector control programme. Additionally, Supplementary Figure 1 has been updated, increasing the range of Wolbachia fitness costs to a minimum value of 30%.
For the global dengue cost model, the text has been updated to clarify that identifying areas to be targeted to reach the WHO goal of reducing global dengue burden by 25% in the most net cost-efficient manner involves considering human population density and per capita GDP in the release area, both factors which have been shown to influence the cost of Wolbachia programmes. Another text addition clarifies that the final decision of which areas to target for Wolbachia replacement should be subject to additional operational, entomological, financial, and political considerations rather than using the maps produced in this analysis prescriptively. Finally, Figure 3 has been updated to correct the axis labels.
All updated code and Supplementary Figures and Tables can be found on GitHub: https://github.com/katietiley/Wolbachia_TPP_PPC.git
See the authors' detailed response to the review by Gabriela Paz-Bailey
See the authors' detailed response to the review by Perran Ross
See the authors' detailed response to the review by Asha Mary Abraham
See the authors' detailed response to the review by Scott O'neill
The Aedes aegypti mosquito is the principal vector of dengue, Zika, yellow fever and chikungunya viruses. Dengue incidence has been rising and the WHO Global Vector Control Response 2017 – 2030 reports an annual 96 million cases, 1.9 million DALYs and 9,110 deaths1. Vaccines are only available for yellow fever and are not currently widely used for dengue, though there are other dengue and chikungunya vaccine candidates in clinical trials2,3. There are no drugs available to combat these infections and so there is a reliance on prevention through vector control. Effective control of this vector is difficult to achieve and sustain given the mosquito’s high reproductive rate and adaptation to urban habitats, with an egg stage that can survive desiccation and a larval phase that can develop in small, temporary water volumes (e.g., water containers and roof gutters). The rapid growth of cities has also favoured this mosquito4. As a result, existing vector control tools alone have generally been unable to sustainably control Ae. aegypti or the diseases it transmits over the long term. A range of novel technologies are under development5, including biocontrol through use of Wolbachia spp. for population replacement or reduction/suppression, the release of genetically modified mosquitoes (such as Oxitec's 1st generation self-limiting technology (1gSLT)6), and other forms of sterile insect technique (SIT).
Ae. aegypti mosquitoes infected with Wolbachia strains show reduced rates of virus dissemination, making them less capable of transmitting arboviruses7. Wolbachia infection is also dominantly maternally inherited and leads to inviable progeny when Wolbachia males and wild-type females mate. This means that Wolbachia can be used to either replace the existing mosquito population with a lower competence phenotype by releasing females (or males and females) or suppress the existing population by releasing only males.
Wolbachia population replacement involves regular releases of Wolbachia-infected mosquitoes into a wild mosquito population over a period of several months. Modelling has shown that once a critical proportion of mosquitoes in the population have Wolbachia, prevalence should continue to increase to fixation without further releases, but below this threshold Wolbachia prevalence may decline (possibly to zero) once releases stop due to fitness costs associated with released mosquito strains8. Operationally, the chance and speed of exceeding this threshold and achieving self-sustaining coverage defined as the percentage of Ae. aegpti population infected with Wolbachia, can be achieved by: increasing the number of releases, decreasing the time gap between releases and increasing the ratio of Wolbachia-infected Ae. aegypti in relation to wild-type Ae. aegypti in each release. All of these options increases cost and can also lead to undesirable temporary increases in the Ae. aegypti mosquito population which should be addressed during community engagement to avoid it becoming could be a key barrier to community acceptability9,10. It should be noted that in practice, Wolbachia frequencies may fluctuate seasonally and still decline to zero after reaching fixation depending on environmental variables such as temperature, rainfall, and physical barriers11,12.
A growing range of entomological, epidemiological and modelling evidence supports the widespread, long-term effectiveness of Wolbachia replacement13–15, and research continues to identify environmental conditions associated with spatially and temporally heterogeneous Wolbachia establishment11. This includes a randomised controlled trial (RCT) of wMel Wolbachia in Yogyakarta City, Indonesia which demonstrated a 77% reduction in dengue incidence and an 86% reduction in hospitalizations16. To date, however, Wolbachia replacement programmes have only been conducted in specific mid-sized cities or specific neighbourhoods of cities. Thirteen countries have implemented replacement programmes at various levels of scale, with 12 through the World Mosquito Program (WMP) and an independent programme in Malaysia17,18. Meanwhile, China (with Ae. albopictus), Singapore, and the USA have so far chosen to use suppression-based programs due to perceived greater compatibility with their existing intensive and long-term efforts to suppress mosquito populations19–21. To date, there have been no large-scale (national or regional), non-donor funded implementations of Wolbachia replacement or suppression approaches.
These novel technologies (Wolbachia replacement, Wolbachia suppression, 1gSLT and SIT) are subjects of ongoing development, evaluation, demonstration and scale-up in various high-burden programmatic and private settings. In addition, there is the potential to combine Wolbachia population replacement with other suppression technologies in ways that may be synergistic from a cost, community acceptance, or dengue incidence reduction perspective9. Specifically, a programme of suppression followed by replacement has the potential to increase the likelihood of successful Wolbachia establishment and reduce the cost of, and risk of mosquito exacerbation associated with, achieving establishment22.
Development and transition to scale of new products and strategies can be accelerated by the development of internationally recognised Target Product Profiles (TPPs) and Preferred Product Characteristics (PPCs) documents23. TPPs provide specific quantitative guidance on the key characteristics a product must (minimum target), or should ideally (preferred target), meet when developed into a deployable mass market product. PPCs identify broader areas of unmet need and aim to stimulate new products or product combinations that can address these needs. In early 2022 the WHO convened a Technical Advisory Group (TAG) to develop a draft TPP for Wolbachia replacement and a draft PPC for a hybrid mosquito suppression then Wolbachia replacement strategy. TAG members agreed that these WHO TPPs and PPCs should closely align with the WHO’s strategy and goals to control dengue globally. As such the WHO’s goal to reduce dengue incidence by 25% by 2030 (2010 – 2020 baseline24) provided a basis to understand the scale and range of settings in which these TPPs, PPCs and the products they ultimately produce are relevant. Computational models can play a key role in the development of TPPs and PPCs due to their ability to generalise beyond areas where data have been collected and make predictions if aspects of the product were to change. Here we describe a dynamic compartmental entomological model and a global geospatial economic model that we developed and used to explore how operational and economic aspects of Wolbachia replacement are likely to change once the technology is used at scale.
The global dengue cost model aims to produce high spatial resolution estimates of the economic costs of dengue that would be averted by Wolbachia replacement. These were conservatively estimated to be composed of the direct medical cost of treatment of dengue patients and emergency (outbreak) vector control costs. In the absence of primary data on willingness to pay for Wolbachia replacement programmes, these averted costs were assumed to represent an appropriate proxy.
A high resolution (5km × 5km at the equator) map of symptomatic dengue case burden was obtained from Bhatt et al.25, which estimates the spatial distribution of the 96 (67 – 196) million episodes estimated to occur each year. An average direct medical cost per symptomatic case (2013 USD) was derived for each country from Shepard et al.26, considering the different costs of hospitalised and ambulatory cases and the country-specific distribution of symptomatic cases among these two different treatment settings. Direct medical costs include the costs of specific medicines and staff time required to treat a dengue patient and a portion of infrastructural costs and is the most relevant measure of what governments need to pay to treat cases of dengue illness each year. All costs were inflated from 2013 to 2020 USD using World Bank country GDP deflators with a maximum capped value of a two-fold increase27.
A literature review on the cost of vector control in dengue endemic countries was conducted and identified studies with national and subnational estimates of vector control costs for 17 countries. Twenty studies included costs of routine vector control activities and seven studies included costs of vector control during dengue outbreaks (supplementary file 1 in Data Availability). All vector control cost values were converted back to local currencies using the exchange rate at the time of the costing, inflated to 2020 using country GDP deflators from the World Bank27, and then converted to 2020 US dollars using 2020 exchange rates published by the World Bank28. To make predictions of per capita routine vector control costs for countries without costing data, a Poisson generalized mixed linear model was fit to the costing data with national GDP per capita (log scale) as a covariate and national-level random effects. Predictions were then made for all countries globally using World Bank GDP per capita figures from 2020. For countries where this data was missing (some small Caribbean and Pacific Island nations), global median GDP per capita was assumed. Of the seven studies identified that included costs of vector control during dengue outbreaks, five studies gathered information on both routine and outbreak vector control activities. We assume that implementing a Wolbachia release program will not avert routine (principally preventative) vector control costs because Wolbachia replacement is unlikely to eliminate dengue in most settings and additional vectors (e.g., Ae. albopictus) and nuisance biting mosquitoes will still drive a need for routine vector control activities. Instead, it was assumed that the implementation of Wolbachia replacement will significantly limit the size of outbreaks and their required vector control response and thus cost. These studies suggested that during outbreaks, the monthly cost of vector control increases by 20–50%. Three scenarios were explored where additional avertable outbreak costs composed 35% of routine monthly vector control costs for a duration of three months every year, with a sensitivity analysis exploring lower (20%) or higher (50%) values.
Total annual averted costs were estimated assuming Wolbachia replacement results in a 70% reduction in symptomatic cases (and their associated costs) and 100% of emergency (outbreak response) vector control costs. This is based on a conservative interpretation of the 77% effectiveness of wMel Wolbachia measured in the Yogyakarta trial16 and the expectation of variable effectiveness across areas with different transmission intensities15. While wMel Wolbachia replacement has been shown to be stable in Aedes mosquito populations for over ten years in Australia14, it is unclear how many future years of averted dengue costs would be appropriate to consider when estimating government or other funder willingness to pay. We therefore estimate total averted costs for three-, five- and ten-year time horizons and assume that these costs represent the maximum price a government or funder would be willing to pay for Wolbachia replacement in a given setting. To quantify uncertainty around these thresholds, the analysis was repeated with values from the upper and lower bounds of the case burden25 and economic burden26 estimates and with 50% and 20% avertable vector control outbreak proportions respectively.
Next we identified which areas (5km x 5km pixels) would need to be targeted to reach the WHO goal of reducing global dengue burden by 25% in the most net cost efficient manner. For a generic environmental intervention, where cost of the intervention only depends on area covered, this would involve targeting areas with the highest density of dengue costs. However, because the cost of Wolbachia programmes have been shown to depend on the human population density and per capita GDP in the release area29, this can change which areas are most important to prioritise from an optimal net cost perspective. To account for these variable implementation cost factors, each 5km × 5km pixel was ranked from highest to lowest based on a benefit (averted medical and outbreak costs) to cost (approximate Wolbachia programme cost estimate based on population density and per capita GDP from Brady et al.29) ratio. For clarity, the approximate Wolbachia programme cost from Brady et al.29 only affects the ranking of pixels (i.e. targeting), not the TPP target cost estimates. Cumulative averted cases were then calculated and pixel selection ended when averted cases first exceeded 25% of the global total. The averted costs in the last, least cost-efficient pixel included in this subset then gave the cost threshold for Wolbachia replacement programmes, i.e., if Wolbachia replacement can be achieved at this cost (or lower) it will be possible, from the cost-efficacy perspective, to implement the intervention in enough areas to reduce the global burden of dengue by 25%. An alternative scenario was also calculated where it was assumed that Wolbachia replacement is only required to account for half of this global target, i.e., a 12.5% global burden reduction. Because such a global targeting approach prioritises countries with higher GDP, we also calculated a scenario where 95% of dengue endemic countries (defined as >10,000 symptomatic dengue infections a year as estimated by Bhatt et al.25) needed to achieve at least a 25% burden reduction through deploying Wolbachia replacement, to explore the cost threshold implication of a much wider deployment with improved equity between countries30.
Since this model was calibrated using data of Wolbachia fixation dynamics from the Yogyakarta RCT16, any features of this RCT influenced the methodology, such as delivery mode (egg releases in release cups) and the number of releases (ranging from nine to 14), therefore, other delivery modes such as releasing Wolbachia-infected adult Ae. aegypti were not simulated. This compartmental mechanistic model follows Aedes aegypti population dynamics at egg, larvae, pupae, and adult stages, with pupae developing into female and male adults in equal proportion and each stage subject to a constant death rate:
O denotes the number of eggs, L larvae, P pupae, M adult males, and F adult females. φ is the daily egg-laying rate of adult females. αO denotes the rate at which eggs develop into larvae and the μO death rate of eggs. Similarly, αL denotes the rate at which larvae develop into pupae and μL the larval death rate and αP denotes the rate at which pupae develop into adults and μP the pupal death rate. μM and μF are the adult male and female death rates, respectively. Survival of larvae to pupal stage is density dependent, and using the flexible formulation proposed by Maynard Smith and Slatkin31, includes the parameter γ which determines the density at which mortality remains proportionate and the parameter β the ‘abruptness’ of density-dependence.
These equations were then further developed to account for Wolbachia deployments, respectively impacting mating and larval survival:
Where, ci denotes the failure rate of cytoplasmic incompatibility for the Wolbachia-infected adult males (MW), and Wolbachia-infected larvae in the wild (LW) also contribute towards larval competition.
While it is important that all wild-hatched larvae are subject to the same density dependence as they are occupying the same habitat, the introduced Wolbachia-infected eggs will be released in their own distinct larval habitat (self-contained release containers), therefore their survival is not impacted by the densities of wild-hatched larvae. The equations of released Wolbachia (rW) are as follows:
OrW denotes the number of released Wolbachia-infected eggs, which is the product of the release ratio (RR) and the equilibrial adult population prior to control (Ḟ + Ṁ). LrW denotes the number of Wolbachia-infected larvae resulting from released eggs, and PrW denotes the number of Wolbachia-infected pupae resulting from released eggs. The aquatic-stage Wolbachia-infected Ae. aegypti that hatch outside of the release containers (subscript ‘W’ instead of ‘rW’) are tracked separately from those which are newly released. The wild-hatching Wolbachia-infected mosquitoes follow these dynamics:
Wolbachia-infected adult mosquitoes comprise those that have emerged from the wild combined with those emerging from release containers:
ε denotes the relative mortality of Wolbachia-infected adult mosquitoes compared to uninfected. A sensitivity analysis explored the impact of Wolbachia infection fitness costs on mosquito population dynamics under Wolbachia release scenarios by varying ε (Supplementary Figure 1). Parameter definitions and values are shown in Table 1.
| Parameter | Description | Value | Reference |
|---|---|---|---|
| φ | Daily egg laying rate of adult females | 500*(1/14) | Otero et al., 200632 |
| Mnull | Male uninfected adults | M + ciMw | - |
| Fall | Total female adults | 1+ F + Fw | - |
| Mall | Total male adults | 1 + M + MW + cMMs | - |
| αO | Daily rate eggs hatch into larvae | 0.5 | Marinho et al., 201633 |
| αL | Daily rate larvae develop into pupae | 0.18 | Marinho et al., 201633 |
| αP | Daily rate pupae develop into adults | 1 | Masters et al., 202034 |
| μO | Daily mortality rate of eggs | 0.01 | Trpis, 197235 |
| μL | Daily mortality rate of larvae | 0.1* | Couret et al., 201436 |
| μP | Daily mortality rate of pupae | 0.1* | Couret et al., 201436 |
| μM | Daily mortality rate of adult males | 1/14 | Yakob et al., 200810 |
| μF | Daily mortality rate of adult females | 1/14 | Yakob et al., 200810 |
| γ | Determines the density at which mortality remains proportionate | 1 | Bellows, 198137 |
| β | Determines the ‘abruptness’ of density dependence | 0.5 | Bellows, 198137 |
| RR | Release ratio of Wolbachia-infected adults compared to total adult mosquitoes | Variable | Estimated |
| ci | Proportion of cytoplasmic incompatibility that fails | 0.012 | Walker et al., 20117 |
| cm | Competitiveness of released sterilised males | 0.5 | Winskill et al., 201438 |
| cv | Proportion of adult population reached by adulticide | 0.141 | Estimated, described in adulticide section |
| ε | Relative mortality of Wolbachia-infected adult mosquitoes compared to uninfected | 1.2 | Joubert et al. 201639 |
| s | Adjustment parameter which matches average seasonal mosquito population to non-seasonal equilibrium mosquito population | 2.09 | Estimated, described in seasonality section |
Wolbachia-infected egg release was also explored after first deploying suppression interventions. The suppression techniques analysed were the release of 1st generation self-limiting technology (1gSLT), sterile insect technique (SIT), Male Wolbachia release, environmental management, larvicides, and adulticides. Each type of suppression was included as a function of time, t, so that a value which influences model dynamics is pulsed at specific times or maintained over a specific period. The efficacy of each method was based on evidence sourced from the literature, selected with a preference for large randomised-controlled trials, however, each suppression method works differently and trials to measure effectiveness vary with study design. Efficacy of a single burst of application was preferable but only found for adulticide. Studies measuring repeated concurrent applications were sourced for 1gSLT, SIT, and Male Wolbachia release, while environmental management and larvicides used an interrupted time series design. The impact of variations in study design are discussed in more detail below.
1gSLT. Release of 1gSLT adult males produce offspring with wild females of which only the males develop to adulthood from the pupal stage. 1gSLT was included in the model by pulsing adult males into a sterile adult male compartment MS weekly, which then contributed to the production of sterile eggs, OS, which developed through sterile larval and pupal compartments, LS and PS, contributing to a density dependant survival function. RRsupp denotes the release ratio for mosquito release suppression techniques. ε denotes the relative mortality of Wolbachia-infected adult mosquitoes compared to uninfected adult mosquitoes.
Parameters for the hypothetical fixed rate efficacy of 20%, 50%, and 80% were calculated by comparing the total adult population at model equilibrium with the minimum adult population reached after five weeks of application. The literature-derived efficacy values were 45% five weeks after the last suppression period and 70% ten weeks after the last suppression period40, calculated by comparing the total adult population at model equilibrium to the total adult population after five- or ten-weeks of suppression which achieved the desired efficacy (summarised in Supplementary Table 1). A caveat of this approach is that the resulting minimum adult population is reached later than five- or ten-weeks, therefore, the maximum efficacy calculated in these scenarios is marginally greater than the literature value stated (shown in Supplementary Figure 2).
SIT. SIT involves releasing sterile adult males which produce sterile eggs (in the same manner of Equation 5) that do not develop further. SIT was included in the model by pulsing adult males into the sterile adult male compartment, MS, which then contributed to the production of sterile eggs which then do not develop further.
Parameters for the hypothetical fixed rate efficacy of 20%, 50%, and 80% were calculated by comparing the total adult population at model equilibrium with the minimum adult population reached after five weeks of application. The literature-derived efficacy values were 49% five weeks after the last suppression period and 77% ten weeks after the last suppression period41, calculated by comparing the total adult population at model equilibrium to the total adult population after five- or ten-weeks of suppression which achieved the desired efficacy (summarised in Supplementary Table 1). A caveat of this approach is that the resulting minimum adult population is reached later than five- or ten-weeks, therefore, the maximum efficacy calculated in these scenarios is marginally greater than the literature value stated (shown in Supplementary Figure 2).
Male Wolbachia release. Male Wolbachia release involves releasing only Wolbachia-infected adult males, resulting in no offspring due to cytoplasmic incompatibility with the local non-Wolbachia-infected females. Male Wolbachia release was implemented in the model by pulsing Wolbachia-infected adult males into the Wolbachia-infected male compartment:
Parameters for the hypothetical fixed rate efficacy of 20%, 50%, and 80% were calculated by comparing the total adult population at model equilibrium with the minimum adult population reached after five weeks of application. The literature-derived efficacy values were 65% five weeks after the last suppression period and 92% ten weeks after the last suppression period42, calculated by comparing the total adult population at model equilibrium to the total adult population after 5- or 10-weeks of suppression which achieved the desired efficacy (summarised in Supplementary Table 1). Similar to 1gSLT, a caveat of this approach is that the resulting minimum adult population is reached later than 5- or 10-weeks, therefore, the maximum efficacy calculated in these scenarios is marginally greater than the literature value stated (shown in Supplementary Figure 2).
Environmental management. Environmental management reduces the amount of egg-laying habitat which was simulated by manipulating the egg production rate:
Parameters for the hypothetical fixed rate efficacy of 20%, 50%, and 80%, and the literature-derived efficacy of 47.4%43, were calculated by comparing the total adult population at model equilibrium with the new population equilibrium reached after applying this technique for the duration of the simulation; this emulates real-world scenarios in which application and outcome are typically long-term (summarised in Supplementary Table 1).
Larvicides. Larvicides were simulated by equally reducing the number of eggs, larvae, and pupae. Because the mode of action is identical and because the best measurement of effectiveness of an intervention that targets the aquatic stages of the mosquito came from a trial of predatory guppies, this effectiveness as measured by 44 was chosen to represent the effectiveness of larvicides.
Parameters for the fixed rate efficacy of 20%, 50%, and 80%, and the literature-derived efficacy of 44%44, were calculated by comparing the total pupae population at model equilibrium with the new pupae equilibrium reached after applying this technique for the duration of the simulation; similar to environmental management techniques this emulates real-world scenarios in which application and outcome are typically long-term (summarised in Supplementary Table 1).
Adulticides. Finally, the deployment of adulticides through fogging and chemical spraying were simulated by pulses which manipulate the mortality rates of adult male and female compartments:
Parameters for the fixed rate efficacy of 20%, 50%, and 80% were calculated by comparing the total adult population at model equilibrium with the minimum adult population after one application. Mani et al.45 reported an initial 94% reduction in mosquito resting density from application of deltacide, a synergized mixture of pyrethroids, after which the population completely recovered within seven days (summarised in Supplementary Table 1). This combination of great suppression and swift recovery could not be replicated in the model by only manipulating adult mortality; this may be because some portion of the reduction in resting density was due to a repellent effect, which has been noted as a possibility by the source paper45 or because recovery was due to recolonisation by neighbouring populations which is not modelled here. To fit this literature efficacy a parameter for 94% mortality rate was first calculated by comparing the total adult population at model equilibrium with the minimum adult population after one application and subsequently a coverage parameter, denoted as cv, was fitted using the 94% efficacy parameter. The highest proportion of coverage was calculated which allowed 80% population recovery within three weeks of suppression using literature-derived efficacy; the assumption of this recovery speed was explored with a sensitivity analysis, shown in Supplementary Figure 3, and found to be minimally affected by changing the number of weeks taken for recovery.
Seasonality is defined by using a normalised and smoothed lowess curve of average monthly precipitation (sourced from www.meteoblue.com) to create a score, bounded by 0 and 1, for a seasonality profile with a distinct wet and dry season each year, produced using data from Rio de Janeiro. Within the model, this score influences γ within the density dependent function. The density-dependent seasonal function of larval survival is:
The seasonality function, fK, returns a precipitation score dependent on time, t, which affects the rate larvae develop and enter the pupal stage. s is a constant, calculated to ensure the average mosquito population in the seasonal model is within 0.5 of the non-seasonal model equilibrium which allows comparability to the non-seasonal analyses as suppression efficacy parameters and functions execute according to this average. This affects all wild-hatched model compartments; however, the released Wolbachia-infected eggs are introduced in containers which isolate them from the limits of rainfall dependent egg hatching and larval growth (seasonal population dynamics shown in Supplementary Figure 4). Placement of the seasonality function within the model was explored (Supplementary Table 2) in addition to the impact of temperature, rather than precipitation, on larval development (Supplementary Figure 5).
Wolbachia coverage data was extracted from the report by Utarini et al.16 using WebPlotDigitzer46 and the intervention cluster-level results used as reference to calibrate the model. Simulations of Ae. aegpti population dynamics were undertaken to investigate the intervention conditions which would produce the desired Wolbachia coverage levels (>95%) in the mosquito population. Specifically, a range of release ratios (0.03 to 0.1, in increments of 0.01) and number of releases (9 to 14, pulsed every 14 days) were explored and their influence on the number of days until target coverage was achieved.
The influence of suppression efficacy (20%, 50%, 80%, and a literature derived efficacy) and the week of switch from suppression to Wolbachia release (1 to 10) was investigated in terms of the minimum release ratio (0.0025 to 0.4, explored in increments of 0.0025) necessary to reach Wolbachia target coverage within six months of the first Wolbachia release.
The seasonality model was run for 18 months and the initial six months burn in period needed for model calibration was discarded; five weeks of suppression followed by five rounds of Wolbachia replacement release were simulated, exploring minimum RR (0.0025 to 0.4 in increments of 0.0025) required to reach target coverage within six months of first Wolbachia replacement release.
To explore how self-sustaining Wolbachia replacement can best be achieved and its sensitivity to various operational parameters we formulate, fit and simulate from an entomological dynamic compartmental model. By calibrating the release ratio parameter, our model showed a good fit to the mosquito release data from the Yogyakarta RCT16 with replacement dynamics and coverage levels proceeding at a similar rate. The model reached 50% coverage after 121 days, compared to an average of 117 days observed in the RCT, and 90% coverage after 180 days, compared to an average of 239 days observed in the RCT (Supplementary Figure 6). As Wolbachia reaches fixation the model slightly overestimates final Wolbachia coverage, likely due to prevalence being suppressed in the RCT due to migration of uninfected adult mosquitoes from outside the release area which were not included in our model and would be reduced when implemented as a wide-scale blanket intervention.
During a Wolbachia replacement programme, our model predicts that the total adult mosquito population experiences a temporary exacerbation above baseline levels, followed by a decline before reaching a new equilibrium once Wolbachia fixation has been achieved (Figure 1A, 1B). Due to the fitness cost of Wolbachia (conservatively modelled to be 20%39, but highly variable depending on environment47), this new equilibrium mosquito population size is predicted to be lower than before Wolbachia release.
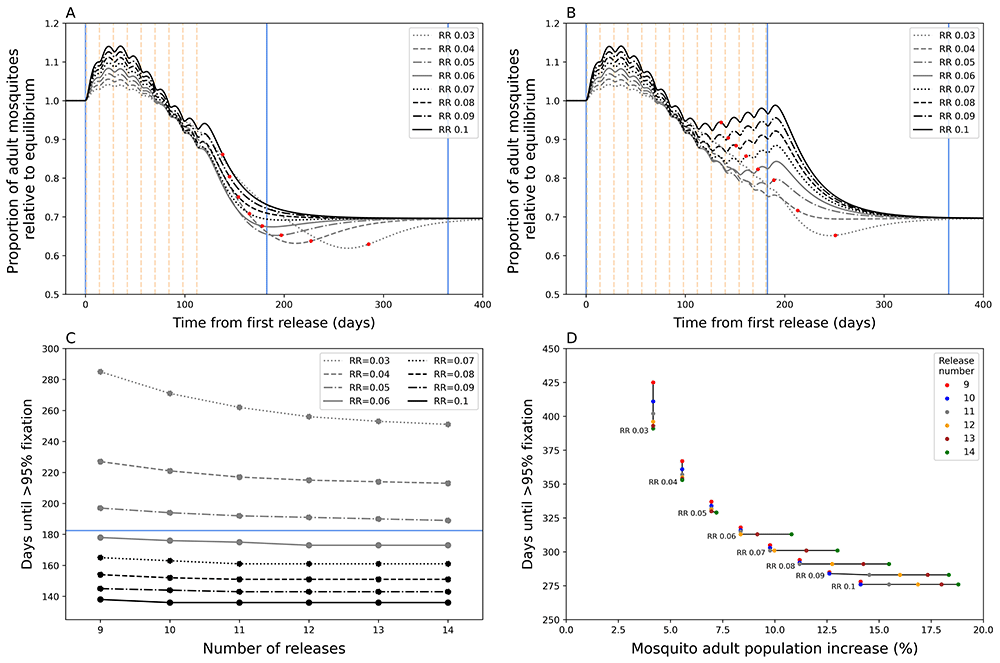
Figures A and B show the total adult mosquito population size over time during Wolbachia replacement after a nine (A) and fourteen (B) release (dotted vertical lines) round programme. Red dots indicate the date at which target coverage (>= 95% Wolbachia coverage) was first achieved. The blue lines show the time points from first release at 0 days, 6 months, and 1 year from left to right. C) Days until Wolbachia-infected adult mosquitoes reach target coverage for different numbers of releases and release ratios (RR). The horizontal blue line indicates 6 months. D) The percentage increase in total mosquito population for different numbers of releases and release ratios. This is calculated as the sum of the peak increase after initial release compared to the pre-release population equilibrium and the peak increase after target coverage is achieved compared to post-fixation equilibrium population, the latter excludes lower RR instances where the population at fixation is lower than the new population equilibrium.
As expected, Wolbachia target coverage can be achieved faster by increasing RR and/or by increasing the number of releases (Figure 1C). Our model shows that increasing RR of each release will reduce time to target coverage more than increasing the number of release rounds, particularly at higher RR values. Above a RR of 0.06, increasing the number of release rounds has little additional effect on time to target coverage. Our model predicts that achieving Wolbachia target coverage within six months of the first release is possible with RR >= 0.06 with >= 9 releases. Higher RR and number of release rounds lead to ever diminishing decreases in time to target coverage. Increasing the RR from 0.06 to 0.1 is only predicted to increase time to target coverage by 40 days (nine release programme, Figure 1C).
Higher RRs and higher release round numbers also lead to disproportionately undesirable temporary exacerbation issues, particularly at higher values (Figure 1A,B,D). A doubling of RR from 0.05 to 0.1 could lead to an approximate doubling of exacerbation (6.96% to 14.11%) under a nine-release programme, but this could be up to 2.6 times more (7.20% to 18.80%) if the number of release rounds were increased to 14. This is because prolonged releases at high RR led to a secondary peak in mosquito abundance that prolongs the period of exacerbation (Figure 1B); in the fourteen-release programme this is still less than the original population, however, increasing number of releases could expect this secondary peak to eventually exceed the prior population size.
Overall, these simulations suggest the importance of balancing speed of Wolbachia replacement with the potentially negative consequences of temporarily exacerbating the mosquito population. In combination with other field evidence, this work supported the TPP’s guidance on “time to achieve target coverage”. The models suggested that a time to achieve coverage of less than 12 months was highly feasible (minimum TPP standard) and that a goal of 6 months (preferred TPP standard) was achievable. To counterbalance the issue of exacerbation, the TPP included a criterion for “community acceptability” that states that any increase in nuisance biting through the chosen release characteristics is “acceptable to local residents”, recognising that the definition of “acceptable” is likely to be highly context specific.
The TAG identified cost as a key reason limiting wider adoption of Wolbachia replacement and therefore a “mature product cost once implemented at scale” criterion was a key feature of the TPP. This cost criterion needed to be low enough to drive innovation and ensure a significant proportion of the global population at risk of dengue can benefit, but not too low as to exclude promising products from further development.
Because detailed data on willingness to pay was unavailable at the time of analysis, we developed a range of scenarios that assume willingness to pay is approximated by the costs of treating dengue cases and of vector control in response to outbreaks over a range of years (Table 2). Each scenario gave a theoretical cost each 5km × 5km area would be willing to pay for Wolbachia replacement. Areas that supported higher costs typically had higher dengue burden but were also heavily influenced by the cost of dengue treatment and prevention.
Wolbachia replacement would need to be at or below this cost to achieve each impact scenario in full. All values show median estimates in 2020 US dollars, brackets show model predicted uncertainty around the true value of this cost threshold at the 95% credible interval level).
We estimate that to achieve a 25% reduction in the global burden of dengue, as per the WHO 2020-2030 goals, using only Wolbachia replacement targeted to the most cost-efficient areas, would require releases across 924,557km2 in 73 countries (Figure 3). This corresponds to 34.7% of the urban (> 300 people per km2) area at risk and just 1.7% of the total area at risk of dengue. If Wolbachia only needs to achieve half of the global 25% reduction, with other interventions responsible for the remaining half, Wolbachia releases would only need to be targeted to 255,459km2 over 47 countries (Figure 2B and 2E). However, because these cost estimates are uncertain and because this approach prioritises high income countries where dengue treatment costs are high, we also include a third and fourth targeting scenario where 25% or 12.5% of the national burden must be reduced for the majority (95%) of dengue endemic countries (Figure 2C and 2F). These scenarios improve equity over dengue-endemic regions. A full list of 2nd administrative units included under each targeting scenario, the costs each will support and additional contextual information (population, density, etc) is included in the following repository: https://github.com/katietiley/Wolbachia_TPP_PPC.git.
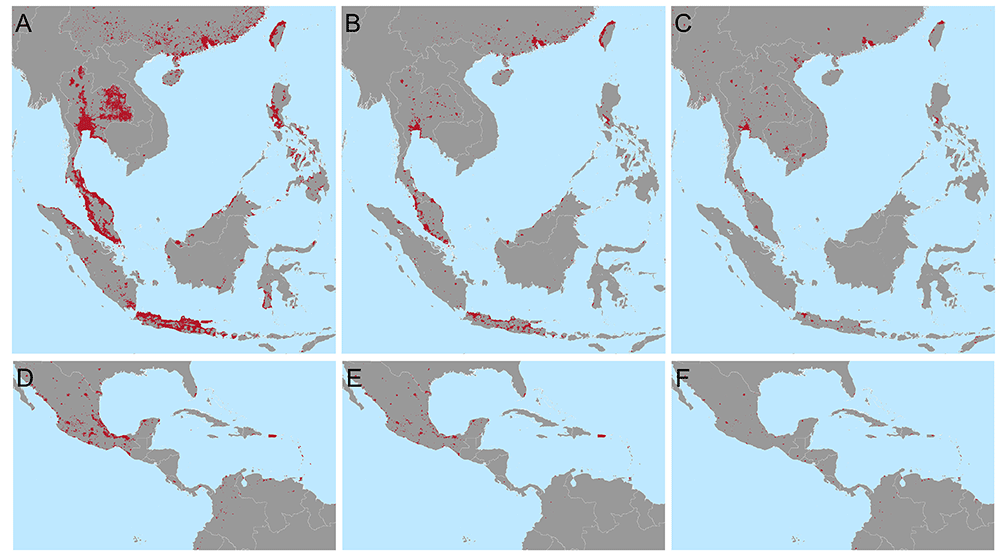
Targeting areas for Wolbachia replacement to meet different global and national goals in Southeast Asia (A–C) and Central America and the Caribbean D–F). Maps show the areas most cost efficient to target (red) to reduce the global burden of dengue by 25% (A and D) or 12.5% (B and E) or the national burden by 25% (C and F) based on the cost of treatment and prevention of current dengue burden. Predictions for other areas and lists of municipalities to be targeted are included in the following repository https://github.com/katietiley/Wolbachia_TPP_PPC.git.
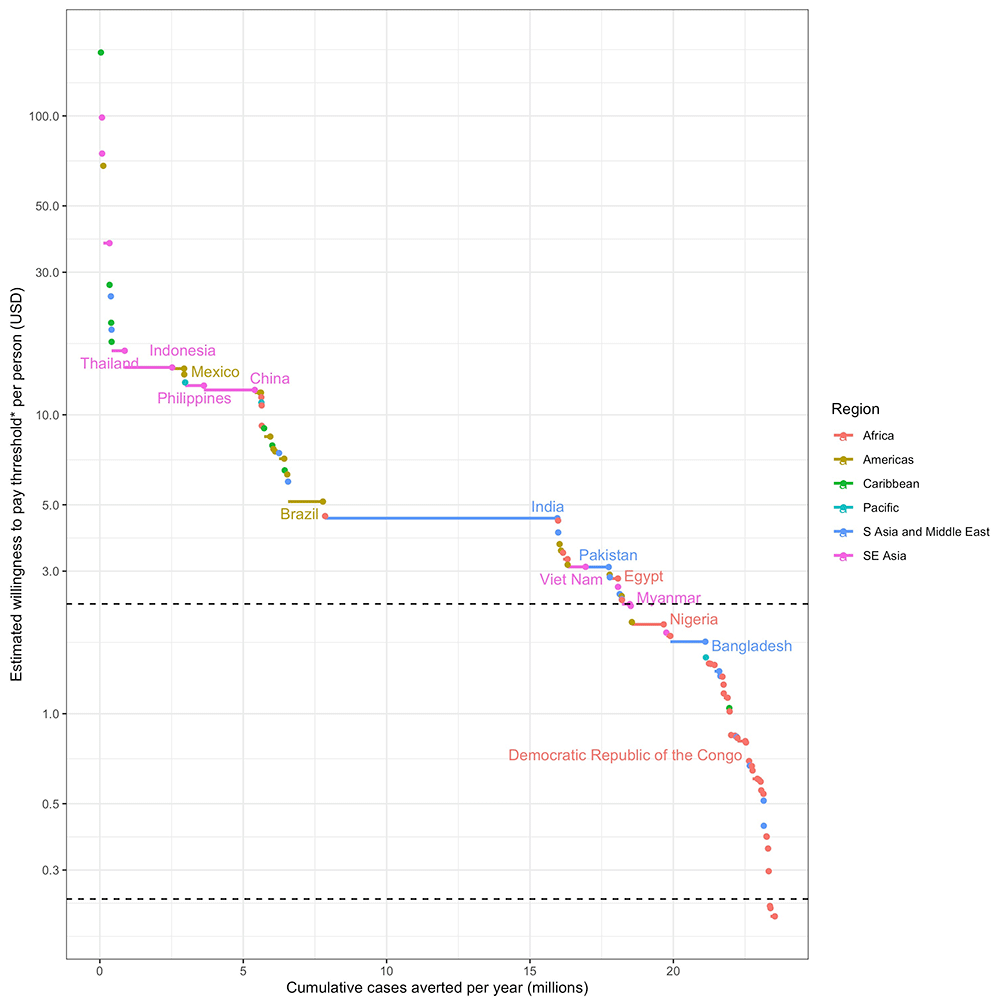
*The willingness to pay threshold is estimated by cumulative medical and outbreak response costs over 5 years for the TPP minimum and 3 years for the TPP preferred criteria. Horizontal dotted lines show the cost thresholds of $2.33 per person and $0.24 per person chosen for the TPP minimum and preferred criteria respectively. Only high burden countries are labelled.
For Wolbachia replacement to be implemented in enough areas to meet these impact targets, the cost of implementation must have the potential to ultimately be reduced to between $7.63 and $0.24 per person covered depending on scenario (Table 2). The cost thresholds identified in Table 2 represent the area with the lowest averted costs (assumed lowest willingness to pay) within the areas needed to reach each impact target. This means that many eligible areas, or even whole countries, could support higher programme costs, but ultimately Wolbachia replacement will need to be implemented at or below this cost threshold in order to reach the impact target. The distribution of these costs and benefits by country is shown in Figure 3 for the 25% global burden reduction impact target. This shows that while globally Wolbachia replacement will need to be achieved for $2.33 per person to meet the 25% impact goal, many countries could support higher costs with many high burden countries able to implement in a wide range of high burden areas above the $10 per person line.
Cost targets become lower (increasingly more ambitious for product development) as impact scenarios become more ambitious or, to a lesser extent, as the accepted duration of benefits becomes shorter (rows and columns in Table 2 respectively). To achieve a 25% national burden reduction in all dengue endemic countries would require a cost target ~ 10x lower than to achieve a 12.5% global dengue burden reduction, emphasising that even higher cost products could still have substantial global impact, but would be less equitable unless subsidised for countries with lower financial capacity for dengue prevention and treatment. Due to high uncertainty in estimates of the true burden of dengue25 and its costs of treatment26 and prevention, uncertainty around these cost thresholds is moderate with higher uncertainty around higher median cost thresholds.
Each of these scenarios and their respective cost targets were presented to the TAG for discussion and selection for the TPP. Recognising that the TPP minimum criteria should reflect the minimum cost for a product to be viable at substantial scale, TAG members selected the $2.33 per person covered target (corresponding to a 25% global burden reduction with five years of benefit, Table 2). This cost needs to include the programme of activities required to reach 90% coverage of Wolbachia in the release areas one year after starting releases. The TPP also makes allowances for a slower programme where 90% coverage is achieved over three years, but this must be achieved at a more stringent minimum TPP cost target of $1.44 per person covered (corresponding to a 25% global burden reduction with three years of benefit, Table 2). However, to challenge developers to meet the more equitable 25% national dengue burden reduction, the TPP preferred cost threshold was set at $0.24 per person covered (corresponding to three years of benefits in this scenario, Table 2). These decisions were also informed by evidence that the current World Mosquito Program cost base for wMel Wolbachia replacement is in the US$5-22 per person range29 with a medium-term goal of achieving Wolbachia replacement for $1 per person48. Given that these TPP targets represent the lowest averted medical and outbreak control costs per person among all areas where releases are required, there are many areas that could support higher programme costs. Therefore, provided developers can demonstrate the prospect of achieving the TPP cost targets in future, there is scope to operate higher cost programmes before these targets are achieved.
In addition to the draft TPP for Wolbachia replacement, the TAG was also tasked to develop a draft PPC for a hybrid mosquito population suppression followed by Wolbachia replacement approach. Reducing the natural mosquito population size allows Wolbachia replacement programmes to achieve higher release ratios or achieve comparable release ratios by releasing fewer Wolbachia mosquitoes. To support the development of the PPC we developed a compartmental entomological model and simulated hybrid strategies with a range of suppression types to answer the questions: can hybrid strategies achieve coverage faster, improve community acceptance and reduce costs relative to a Wolbachia replacement programme alone?
Development of this model first involved fitting the model to the literature-derived efficacy estimates, which differ for each suppression method. Study design influences measurement of maximum suppression efficacy, time taken to reach maximum suppression, and time taken to recover to pre-suppression levels, which were all considered when fitting the model and making predictions for a standardised single application programme. When comparing a single suppression application with literature-derived efficacy adulticide achieves the greatest suppression but rapidly returns to pre-suppression levels (Figure 4). Other methods take longer to reach peak effectiveness, but also have longer durations of effectiveness, particularly insect release methods. Male Wolbachia release is the most effective insect-release method but 1gSLT has a longer-lasting effect. Finally, environmental management and larvicides were estimates to result in the least suppression when applied over a short time period as they typically require long consistent periods of application to reach maximum suppression efficacy. With reductions in adult mosquito population size in the range of 8.18 – 43.51%, from a single application all methods of suppression were predicted to remove the ~1–10% mosquito population exacerbation seen in replacement only programmes.
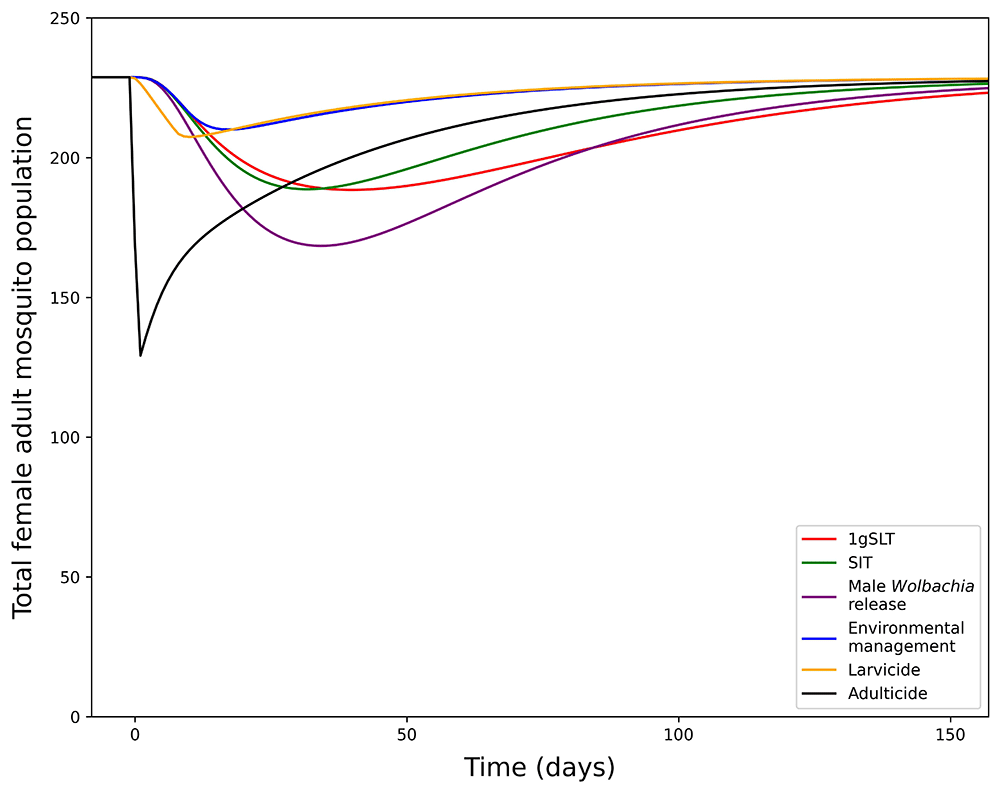
Showing the total adult female mosquito population dynamics under 1-week suppression application with different methods.
Our model predicts that a prior suppression programme of five weekly rounds could reduce the number of Wolbachia mosquitoes required to reach target coverage within 6 months by 16–81% depending on suppression method used (Figure 5A, comparing literature-derived estimates). All insect release-based suppression methods gave greater reductions in required Wolbachia mosquitoes than conventional methods. This superiority is maintained even if the peak effectiveness is standardised across different methods of suppression (Figure 5A), suggesting longer-lasting suppression methods are preferable for hybrid approaches. It may seem counterintuitive to use mosquito killing methods at the same time as Wolbachia mosquitoes are being released, but if these mosquito killing methods do not disproportionately affect Wolbachia mosquitoes relative to the wildtype (as assumed in our model), suppression will still reduce the overall number of Wolbachia mosquitoes required for replacement. Among conventional suppression methods, adulticide outperformed environmental management and larvicide with reductions in required release ratio of 39%, 19% and 16% respectively, considering literature-derived efficacy.
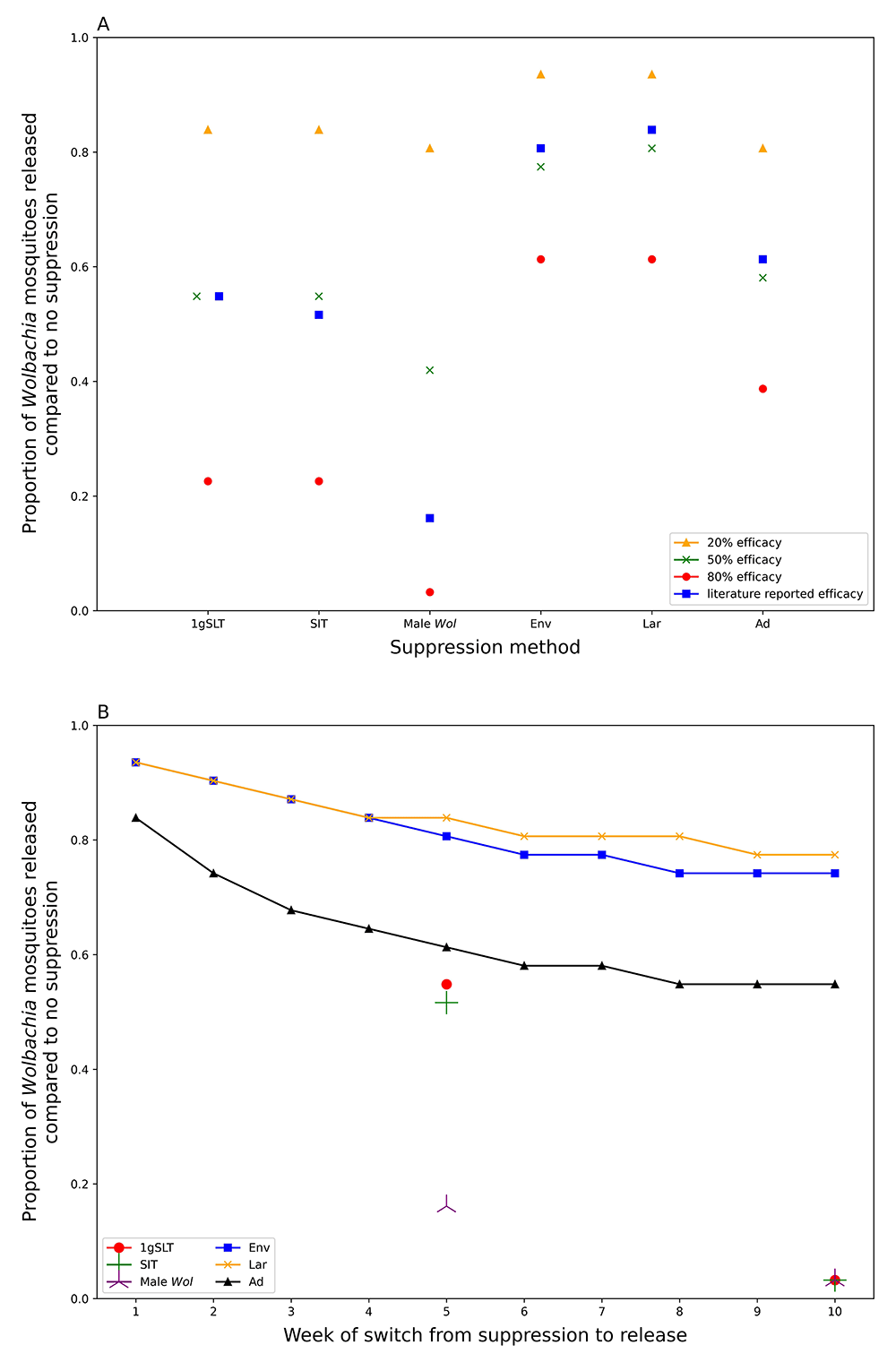
Replacement with no prior suppression requires a Wolbachia release ratio of 0.0775 to achieve target coverage (95%) within 6 months of first release. A) shows the proportional reduction in required Wolbachia release ratio following a 5-week suppression programme with different methods. B) shows how this proportional reduction in required Wolbachia release ratio declines with increasing rounds of suppression. All suppression methods use literature-derived efficacy; 1gSLT, SIT, and Male Wolbachia release only show two data points because the literature calculated efficacy at 5- and 10-weeks.
Similar results were found when the number of Wolbachia release rounds were reduced (as opposed to reducing the release ratios per round) suggesting programmes could realise this benefit by reducing the number or density of Wolbachia releases. Conversely, programmes could choose to release the same number of Wolbachia mosquitoes, but now at much higher release ratios which would achieve Wolbachia target coverage faster. Insect release suppression methods could be used to decrease the time to target coverage by up to 80%, while conventional methods would only marginally improve speed, or not at all in the case of environmental management (Table 3).
* Based on a programme that achieves the TPP minimum target cost of $2.33 per person. Maximum cost savings in the replacement phase assumes suppression has no cost or is an in-kind contribution. ** relative to a baseline programme with nine releases at a relative ratio of 0.09 achieving target coverage (95%) in 215 days.
| Method of suppression | Maximum percentage cost reduction | Maximum cost savings in the replacement phase* ($ per person covered) | Percentage reduction in days to achieve target coverage** |
|---|---|---|---|
| 1gSLT | 18.1 – 50.2 | $0.42 – 1.17 | 83.4 |
| SIT | 17.4 – 48.3 | $0.41 – 1.13 | 82.5 |
| Male Wolbachia release | 17.4 – 48.3 | $0.41 – 1.13 | 82.5 |
| Environmental management | 3.8 – 10.7 | $0.09 – 0.25 | 0.0 |
| Larvicide | 6.4 – 17.8 | $0.15 – 0.42 | 11.3 |
| Adulticide | 7.7 – 21.4 | $0.18 – 0.50 | 18.5 |
All hybrid programmes had the potential to offer cost savings over replacement alone, depending on the cost of suppression. Mosquito release suppression methods could reduce costs by 18–50% and conventional methods by 4–21% (Table 3). If the baseline replacement programme can meet the TPP minimum cost target of $2.33 per person, then these percentage reductions would translate to reductions of $0.41 – $1.17 per person for mosquito suppression methods and $0.09 – 0.50 per person for conventional methods. This, however, does not take into account the costs of suppression. A different interpretation of these results would be: if suppression can be achieved for less than these costs, then a hybrid programme will cost less than replacement alone. This might refer to specific circumstances when suppression might be achieved at negligible cost, for example, if it’s already part of a vector control programme. These are ideal situations where the resources for suppression are already in place, however, in most cases the potential savings achieved by a hybrid suppress then replace programme won’t justify the additional cost of implementing a novel suppression programme. Even if the costs are equivalent, a hybrid programme may be considered more acceptable from a community perspective due to prevention of the temporary increase in numbers of biting female mosquitoes. The mosquito-release suppression techniques (i.e., 1gSLT, SIT, Male Wolbachia release) may also lead to a temporary increase in mosquito population and would require separate community engagement activities to emphasise that the released males do not bite or increase the risk of infection.
Longer suppression campaigns give diminishing returns when used as part of a hybrid approach (Figure 5B). The greatest benefits, in terms of reducing Wolbachia release requirements, are seen within the first few weeks of suppression with decreasing benefits beyond five weekly rounds of suppression. This effect is more pronounced for insect release suppression methods that see most of their benefits delivered from a one- to three-week suppression programme, while for conventional methods there may still be some benefit in continuing suppression for up to eight weeks (Figure 5B).
The primary role of these modelling results in developing the PPC was to clarify the likely benefits of the hybrid approach. Namely that a hybrid approach could achieve Wolbachia coverage faster, with higher community acceptance and/or at a lower cost. Citing this modelling input the TAG concluded in the PPC that a range of suppression methods can be considered for combination with Wolbachia replacement to achieve potential benefits of faster achievement of coverage by Wolbachia, higher community and programmatic acceptance and lower cost. The draft PPC states that “trials of a hybrid approach would test the expected benefits of conducting suppression followed by replacement and that modelling thus far suggests that suppression methods involving insect releases will generally reduce the intensity of the Wolbachia replacement programme more than other methods”. It was also agreed that modelling would be a useful tool for prioritising intervention combinations for field trials and could be used to directly inform trial design. Finally, the PPC recognises that hybrid approaches may require additional logistical and practical complexities over replacement alone, particularly for mosquito release suppression methods that may require additional regulatory approval. This may mean that rather than hybrid approaches superseding replacement-only approaches, their use may be restricted to areas where replacement-only cannot meet speed, acceptability, and cost goals.
Extension of this modelling work to account for seasonal variations in mosquito population size with the aim of optimising the seasonal timing of replacement-only and hybrid approaches was also requested. Since the original PPC meeting, our model has been expanded to include a typical seasonal profile. Our model assumes mosquito population sizes closely follow variation in precipitation with a 41-day lag and a peak population size ~ 3 times the dry season minimum, consistent with various field observations49 (Supplementary Figure 4, seasonality function sensitivity analysis in Supplementary Table 2).
We predict that the optimal time to begin replacement-only or hybrid programmes is just before the seasonal lowest point in mosquito abundance (Figure 6). The release ratio required for Wolbachia fixation in a replacement only programme mirrors precipitation (and thus wild type mosquito population) dynamics, with a short lag.
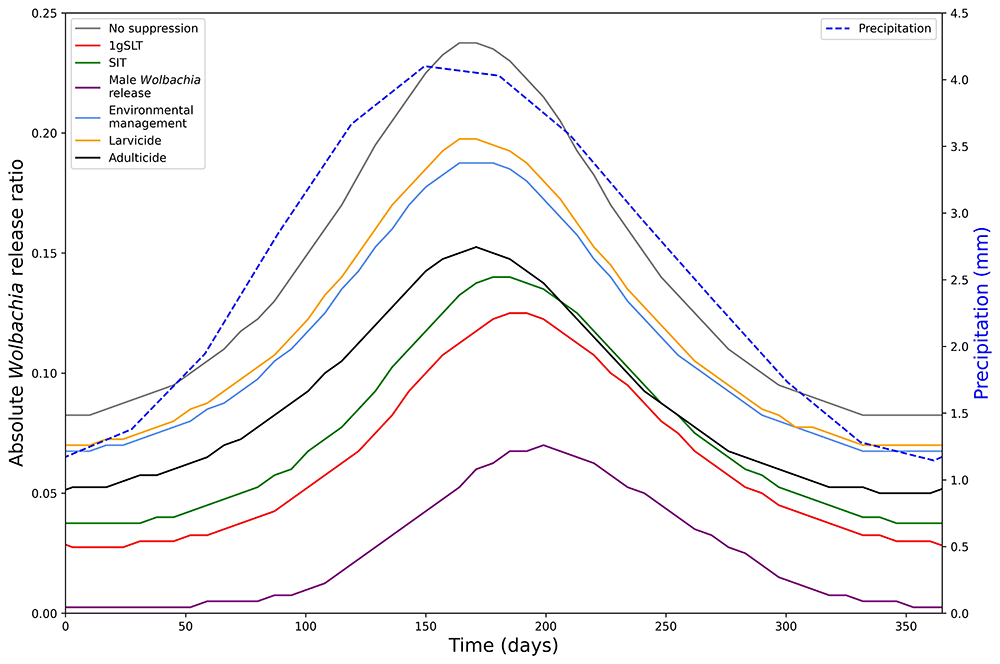
Minimum release ratio for a Wolbachia replacement programme to reach target coverage (95%) within 6 months of first release after a 5-week suppression programme with different methods. Required release ratio is relative to the annual mean (long-term average) wild type mosquito population. Seasonality in mosquito abundance is simulated by precipitation-dependent larval development.
When mosquito populations fluctuate throughout the year, the timing of Wolbachia replacement has a large effect on the number of Wolbachia mosquitoes that need to be released to reach fixation. Starting the replacement programme at the optimum time can reduce the number of Wolbachia mosquitoes by 65.26% compared to the least optimal time. The seasonal scenario also follows the prioritisation of suppression methods observed in the non-seasonal analysis when using a hybrid suppression-then-release approach with male Wolbachia release most effective and larvicide least effective. Furthermore, at the optimal time, in the dry season, the hybrid approach reduces the required Wolbachia release ratio by up to 93.94% compared to replacement alone, whereas at the least optimal time, in the wet season, the hybrid approach only reduces the required Wolbachia release ratio by up to 64.21%. Finally, because of their delayed effects the insect-release suppression methods (1gSLT, SIT, Male Wolbachia release) allow a hybrid strategy to remain effective for longer in the early stages of the wet season, so may be a better choice for areas where the timing of mosquito seasonal cycles is less predictable.
Mathematical and geostatistical models can make important quantitative and qualitative contributions when developing TPPs and PPCs. Here we show that models can: i) identify important trade-offs, such as the time taken for Wolbachia to reach target coverage and the temporary exacerbation in the mosquito population, ii) quantify threshold criteria, such as the $2.33 per person Wolbachia replacement cost target, iii) predict characteristics of a product in new areas and at broader scales than it is currently implemented, such as to meet the WHO 25% global burden reduction targets and iv) understand synergies and antagonisms between combinations of products that have not yet been tested, such as a hybrid suppress then replace approach. The population dynamics shown in our model are consistent with previous modelling and field studies showing temporary population exacerbation and successful fixation within one year9,50,51; this evidence supports the credibility of our findings quantifying the difference between scenarios with varying release ratio and release number.
Broad community acceptability of Wolbachia replacement will clearly be a critical aspect of achieving implementation at the scale envisioned by these TPP and PPC documents. The success of current replacement programmes has been underpinned by extensive community engagement activities52,53 and other countries (Singapore20 and China19) have chosen to use Wolbachia for suppression only, in part, due to concerns over any increases in mosquito abundance. Here we show temporary increases in mosquito abundance can be minimised or avoided entirely by using lower Wolbachia release ratios, timing releases to coincide with the dry season, or conducting a prior suppression campaign in a hybrid approach. These steps may, however, involve additional programmatic complexity and likely cost. More work is needed to better understand how mosquito abundance relates to community acceptability in different contexts and how such barriers can be overcome with different release intensities, timings, and hybrid approaches. One alternative use case, considered in the TPP, is to conduct longer lower intensity Wolbachia replacement releases to reach an initial target coverage of 50%, after which gaps in coverage are gradually infilled by movement of mosquitoes and auto-dissemination of Wolbachia. These lower density releases may have significant cost advantages and could be a more acceptable method of dissemination over broad areas where faster implementation is a lower priority. Such a strategy would, however, take longer, be dependent on patterns of mosquito movement and may be limited by environmental barriers to mosquito spread54. Development of spatial models of mosquito movement and dengue spread could help identify where additional release points may be necessary, target initial release points to high-risk areas and quantify the collateral benefit in disease reduction in neighbouring areas55,56.
Cost continues to be a barrier to wider adoption of Wolbachia replacement when its high costs but long-term benefits are compared to lower cost but short acting suppression methods, despite differences in the evidence base underpinning these benefits29. A key strength of our analysis was to link TPP cost targets to conservative estimates of averted costs based on direct medical costs and emergency vector control expenditure over limited timeframes. This was critical to identify geographic differences in cost targets between, but also within countries. Pairing this analysis with high resolution global burden and cost maps identified cost targets that are compatible with wider international goals and equitable across a range of settings24. Work is currently underway to validate our approximation of willingness to pay for Wolbachia replacement through surveys targeted to key stakeholders in state and federal governments. The maps and models generated in this work could be adapted for planning national Wolbachia replacement campaigns and, in particular, could inform how re-use of release resources, variable pricing models, financing and slower release campaigns could be used to meet the TPP cost targets in even the most challenging countries29. Some of this functionality is already available in the freely available Wolbachia Decision-Support tool (https://wolbachia-tool.netlify.app/tool#map) which makes use of the outputs of this analysis along with other geospatial layers. It is important to clarify that these maps should not be used prescriptively, but rather give an indication of the kinds of areas that are likely to be most cost efficient to target, with the final decision on which areas are targeted for release subject to additional operational, entomological, financial and political considerations.
This analysis predicts that Wolbachia replacement releases over 924,557km2 could lead to a 25% reduction in dengue burden globally, averting US$3.05 billion (2.62 – 3.96) worth of medical and outbreak response costs per annum. This may appear ambitious for a minimum product but can be understood in the following context. Although the lowest averted cost/person covered in this area is predicted to be US$2.33, all other release areas could support substantially higher costs (Figure 3). This means that a Wolbachia replacement product would still meet the TPP targets if initial programme costs were higher and if the product has the potential to reduce costs down to the $2.33 target. It is important to note that the TPP only requires that the product is suitable and available everywhere within the target areas, not that it is necessarily implemented in all suitable areas. In practice, commercial considerations, including the need to build capacity, to access funding that is incremental to the current routine control budgets, and to compete and combine with other methods, will limit the rate of uptake and the ultimate scale of deployment achieved.
Hybrid approaches offer one promising option for increasing speed, reducing cost and increasing the acceptability of Wolbachia replacement. The models presented here and the wider evidence provided to the PPC support field trials of hybrid approaches as a next logical step. These models can guide the prioritisation of suppression methods, trial sample size calculations and suggest how effectiveness should be measured. We predict that insect-based suppression methods (1gSLT, SIT, Male Wolbachia release) will be more effective than conventional suppression tools, but also outline a limited cost window which may be challenging for insect-based suppression methods to achieve. Investment in new infrastructure to conduct insect-based suppression may not be justified for a one-off suppression, but between overlapping resource requirements for suppression and replacement, ongoing use post-replacement (e.g., outbreak control or to achieve dengue elimination) and a continued drive to lower costs of mosquito suppression57, this investment cost may be justified. Intervention developers and countries must ultimately decide how to balance cost and efficacy when considering hybrid approaches. Timing replacement to coincide with the seasonal low point of mosquito abundance is an alternative low-cost hybrid approach and would be a useful addition to trials of hybrid approaches. More generally, our results also suggest that suppression methods that have a longer residual effect are likely to be more beneficial in a hybrid approach. This would suggest some emerging vector control methods, including targeted indoor residual spraying (TIRS)58,59 and Oxitec’s second-generation Friendly™ mosquito technology60 that allows male survival, would also be strong candidates for a hybrid approach and should also be considered for inclusion in modelling and potentially in hybrid field trials.
These models and the results they generate are not without their limitations and clear communication of these limitations was an important part of their use for the TPP and PPC. Our models of Wolbachia replacement do not include any spatial, temporal (beyond seasonal) or stochastic heterogeneities that may mean our model overestimates the speed to achieve fixation and target coverage, particularly in the latter stages (Supplementary Figure 2). We also do not account for a natural wild type egg bank emergence which can dilute Wolbachia release ratios, possibly accounting for the low RR values estimated in these analyses. The Wolbachia replacement system targets Ae. aegypti, which is the major vector of dengue, but Ae. albopictus can be locally important and the need for a Wolbachia replacement product to control both species is not considered in this modelling. Ae. albopictus and other mosquito species are assumed to be controlled by routine vector control programmes which would be ongoing in parallel with any Wolbachia Ae. aegypti replacement or hybrid control programmes. While this modelling was designed to be generalisable to successfully inform the TPP and PPC documents, Wolbachia replacement programmes should be context specific. Therefore, when considering extrapolation in new environments, successful long-term establishment will be context dependent as many variables may vary depending on environmental factors11,12 which could not be simulated in this model, for example, mosquito entomological parameters and Wolbachia density is influenced by temperature61, while physical barriers such as highways may affect mosquito dispersal54. There remain large gaps globally in data on the cost of dengue treatment and prevention and no comprehensive cost estimates for Zika, chikungunya or yellow fever, all of which Wolbachia will provide some efficacy against. Cost estimates are therefore generally conservative and should not replace primary data on willingness to pay or more detailed cost-benefit analyses when considering programmes in any one given country. We also recognise that only one modelling group was included for this TPP and PPC and that inclusion of multiple modelling teams can help better represent the structural uncertainty of models and their interpretation when deciding between policy options62. Fitting each suppression method to literature reported effectiveness estimates was challenging due to incomparable ways in which suppression was implemented and evaluated, and therefore the modelling outputs for each method may not be representative. In particular, our chosen source of evidence for adulticide suppression reported a 94% followed by a return to pre-suppression population within 7 days45, a rate of rebound that our model was unable to replicate from newly emerging adult mosquitoes alone, thus we had to assume that the 94% effectiveness was only achieved in a fraction of the overall mosquito population. Moreover, this efficacy is higher than generally expected for outdoor space-spraying in urban environments in practice63. Additionally, suppression efficacy is highly context-dependent; to mitigate this we modelled an hypothetical 20%, 50%, and 80% suppression efficacy to allow clearer comparison between suppression techniques. Our hybrid approach results also assume that the suppression method acts independently of any other forms of vector control already in use in the area. Suppression will likely be reduced if the method of suppression (or similar methods that use the same modes of action or insecticides) is alreadyroutinely used.
In this paper we show the value dedicated modelling research can add to the development of TPPs and PPCs. By making the data, code and fitted models freely available to accompany the TPP for Wolbachia replacement and PPC for the hybrid approach, product developers are able to continue to use and adapt them to steer the development of a range of Wolbachia products to meet the rising challenge of global dengue control.
No ethical approval was necessary as all data used was publicly available.
GitHub. Using models and maps to inform Target Product Profiles and Preferred Product Characteristics: the example of Wolbachia replacement. DOI: https://github.com/katietiley/Wolbachia_TPP_PPC.git
This project contains the following data:
- All data and code used for each of the models used in this analysis and the predictions made by these models
- Supplementary file 1 and all supplementary figures referred to in the main text
Data is available under the MIT Licence.
We acknowledge Marjorie Opuni-Akuamoa for her contributions towards the cost of dengue vector control systematic review. We also acknowledge Raman Velayudhan for his guidance on experimental design, communication of results and for facilitating the modelling inputs to the TPP and PPC processes.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Dengue and other arbovirus diagnostics and research including cell culture, serology, molecular assays.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology
Competing Interests: I am the heavily involved in Wolbachia replacement technology implementation through my role in the World Mosquito Program.
Reviewer Expertise: Wolbachia biology and its use for disease control
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Hancock PA, Sinkins SP, Godfray HC: Strategies for introducing Wolbachia to reduce transmission of mosquito-borne diseases.PLoS Negl Trop Dis. 2011; 5 (4): e1024 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: I am actively involved in research on Wolbachia population replacement programmes in Aedes mosquitoes, including lab experiments and analysis of field data. My comments focus on Wolbachia and mosquito biology and the outcomes of release programmes. I am not a statistician and am not qualified to comment on the equations behind the models, only their biological relevance.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Soh S, Ho SH, Seah A, Ong J, et al.: Economic impact of dengue in Singapore from 2010 to 2020 and the cost-effectiveness of Wolbachia interventions.PLOS Glob Public Health. 2021; 1 (10): e0000024 PubMed Abstract | Publisher Full TextCompeting Interests: I am the CEO of the World Mosquito Program, a not-for-profit that has developed and implements Wolbachia replacement technologies. My conflict is non-financial but relevant given that the paper describes the use of our technology
Reviewer Expertise: Wolbachia biology and its use for disease control
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 3 (revision) 31 Oct 24 |
read | |||
|
Version 2 (revision) 28 May 24 |
read | read | read | |
|
Version 1 12 May 23 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)