Keywords
Pregnancy, Anemia, Iron, Iron-deficiency, Birth weight, RCT
Anemia affects 40% of pregnant women globally, leading to maternal mortality, premature birth, low birth weight, and poor baby development. Iron deficiency causes over 40% of anemia cases in Africa. Oral iron supplementation is insufficient for Low-and-Middle-Income-Countries (LMICs) to meet current WHO targets. We hypothesized that a single intravenous dose of Ferric Carboxymaltose (FCM) may be more effective than oral iron treatment for anemia recovery, particularly in these settings where women present late for antenatal care.
This is a two-arm parallel open-label individual-randomized controlled trial in third trimester, in malaria Rapid Diagnostic Test-negative pregnant women with moderate or severe anemia - capillary hemoglobin <10 g/dL – who are randomized to receive either parenteral iron – with FCM – or standard-of-care oral iron for the remainder of pregnancy. This is the sister trial to the second-trimester REVAMP trial, funded by the Bill and Melinda Gates Foundation (trial registration ACTRN12618001268235, Gates Grant number INV-010612). In REVAMP-TT, recruitment and treatment are performed within primary health centers. The trial will recruit 590 women across Zomba district, Malawi. The primary outcome is the proportion of anemic women - venous hemoglobin <11 g/dL - at 36 weeks’ gestation or delivery (whichever occurs first). Other pre-specified key secondary clinical and safety outcomes include maternal iron-status and hypophosphatemia, neonate birth weight, infant growth and infant iron and hematological parameters.
This study will determine whether FCM, delivered within primary health centers, is effective, safe and feasible for treating moderate to severe anemia in third-trimester pregnant Malawian women. This intervention could have long-term benefits for maternal and child health, resulting in improved survival and child development.
Pregnancy, Anemia, Iron, Iron-deficiency, Birth weight, RCT
In response to reviewers suggestions and after considering other aspects of the statistical analysis of the trial, the following modifications were made to the manuscript:
- Addition of text “As REVAMP-TT is an effectiveness trial, the standard-of-care oral iron arm will be provided to participants following the established recommendations issued by the Malawi Ministry of Health”, page 12, under Trial design.
- Removal of “malaria positivity by RDT”, page 14, under Safety Outcomes.
- Addition of revised statistical analysis plan “In the event of non-convergence, we will use alternative models”, page 16, under Main analysis of the primary outcome.
- Addition of revised statistical analysis plan “In the event of non-convergence, we will use alternative models”, page 16, under Analysis of secondary outcome(s)
- Two instances of removal of “and malaria RDT positive”, page 16, under Analysis of safety outcome(s)
- Addition of “Using a model similar to the main analysis model of the primary outcome described above, the treatment effect at 36 weeks’ gestation will be examined for the primary outcome”, page 16, under Additional analyses.
- Addition of “(gravidity (primigravida vs. multigravida), baseline HIV status (positive vs negative), baseline severe anaemia status (yes vs no severe anaemia), baseline iron-deficient status (yes vs no ID), baseline iron-deficient anaemia status (yes vs no IDA), baseline inflammation status (yes vs no elevated CRP)”, page 17, under Additional analyses.
- Correction of follow-up dates from “June” to “May”, page 17, under Trial status.
See the authors' detailed response to the review by Adam Lewkowitz
See the authors' detailed response to the review by Sue Pavord
Anemia during pregnancy is a critical global health problem, affecting almost 40% of pregnant women worldwide, with the highest rates being found in Africa and Asia1. It is associated with significant risks for both the mother and the child, including maternal mortality, prematurity, low birth weight, and impaired development2–4. Control of anemia in women is a key 2025 global nutrition target5.
Oral iron supplementation during pregnancy has been shown to be beneficial and safe6. A trial in 470 Kenyan pregnant women demonstrated the benefits of oral iron supplementation in reducing the risk of low birth weight, lengthening gestation duration, and reducing the risk of premature birth7. Global recommendations for the management of anemia in pregnancy in Low- and Middle-Income Countries (LMICs) are that women be treated with high dose of daily oral iron supplementation for three months (120 mg of elemental iron)8,9. However, uptake and adherence to oral iron therapy during pregnancy are inadequate10. Moreover, even when delivered, oral iron frequently fails to correct anemia in routine practice11. These challenges need to be addressed to ensure adequate uptake and adherence to oral therapy during pregnancy if the 2025 global nutrition target of a 50% reduction of anemia in women of reproductive age is to be achieved.
Ferric carboxymaltose (FCM) is an intravenous iron therapy that has revolutionized the treatment of iron deficiency anemia12. It can be administered over 15 minutes and at doses up to 1000 mg, allowing for a total dose of iron replacement to be achieved in a single visit12. FCM has been available in Europe since its approval in 2007 and in the USA since 2009, and over 50 countries currently market it12. FCM has become a widely used treatment for anemia in pregnancy in developed countries13–15. However, little data exists on the feasibility, safety profile and clinical efficacy of delivering FCM in LMICs, including its effects on maternal anemia, and the effects on postpartum health or long-term infant growth and development.
In our two-arm open-label individual-randomized controlled trial (REVAMP) of moderate to severe anemic women in the second trimester of pregnancy in the Blantyre and Zomba district, Malawi, we found that compared to standard-of-care oral iron, FCM could be safely delivered in 862 women with no serious infusion reactions and no increase in adverse events16. Although FCM reduced iron deficiency and iron deficiency anemia across timepoints from four weeks post infusion, week 36 gestation, delivery, and four weeks postpartum, a statistically significant reduction in anemia was not observed except for four weeks post-infusion (77% FCM versus 84% standard-of-care). The feasibility of implementing such an intervention within a primary care health setting is being explored within an associated project to ensure target populations that may benefit most can receive it17. Our team conducted the REVAMP trial18,19, funded by the Bill and Melinda Gates Foundation (trial registration ACTRN12618001268235, Gates Grant number INV-010612), which was published this year16. This trial looked at the safety and efficacy of delivering Ferric Carboxymaltose to anemic women in their second trimester of pregnancy, with the intervention being delivered at the research sites in Zomba and Blantyre, southern Malawi. At four weeks post-intervention women in the IV-iron group were significantly less anemic than those in the oral iron arm, but this was not carried to the primary outcome at 36 weeks gestation or had any significant impact in key outcomes such as maternal anemia at delivery and baby birth weight16. Given that the third trimester is when fetal iron requirement reaches its peak, REVAMP-TT has the potential to target the most important period for iron availability during pregnancy20.
In REVAMP-TT, we will determine the effectiveness of intravenous iron – given as FCM – once during the third trimester (27–35 weeks’ gestation) on anemia recovery by 36 weeks’ gestation or at delivery, whichever comes first, compared to standard-of-care oral iron. We hypothesize that in Malawian pregnant women with anemia in the third trimester, compared to standard-of-care, treatment with a single dose of FCM will be superior in terms of a reduction in anemia prevalence prior to or at delivery. We also hypothesize that FCM will have positive impacts on other maternal and neonatal outcomes and will be safe.
National Health Sciences Research Committee of Malawi Approval – NHSRC REF. Number: 20/11/2622. Human Research Ethics committee at WEHI – HRE REF. Number: 20/25.
This manuscript summarizes the trial protocol and statistical analysis plan (SAP).
In short, pregnant women attending antenatal care at eight primary health centres in Zomba district, Malawi (Figure 1), will be prescreened. Women in their third trimester, with negative malaria rapid diagnostic tests (RDT) and a capillary hemoglobin of 10 g/dL or less will be invited to be enrolled in the trial (Figure 2). Women will be randomized to receive either intravenous FCM or standard-of-care oral iron. All trial procedures and follow-up times are detailed in Table 1. This protocol describes the main trial looking at pregnant women in their third trimester and following them until one-month postpartum, after which we will report on the primary and secondary objectives. Follow-up of mothers and infants from one-month postpartum to 12 months postpartum consists of a range of exploratory economic, biological, cognitive and clinical outcomes. These analyses are beyond the scope of this protocol, as are the exploratory outcomes collected up to 12-months postpartum and will be reported separately. The trial design is summarized in Figure 2.
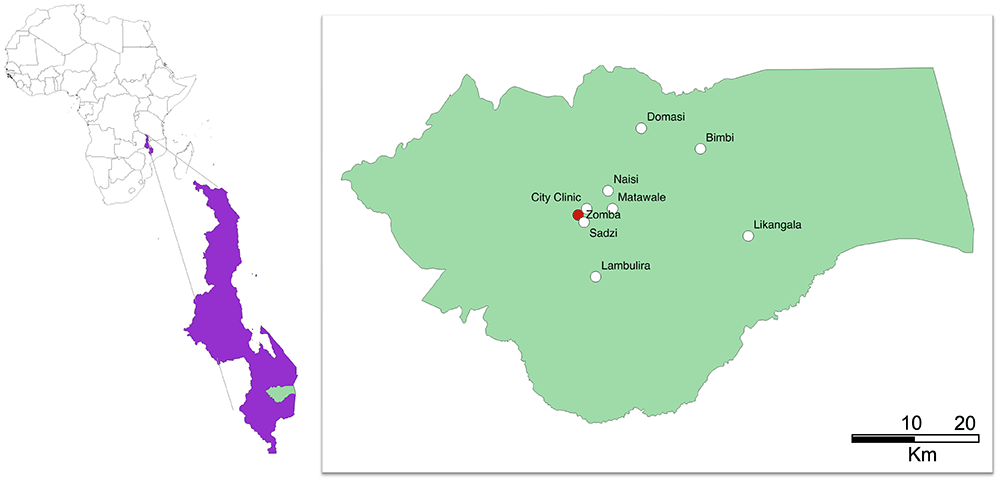
Figure prepared in QGIS2.10.

Trial design with visit timings, visit numbers (standardized to match those of REVAMP trial – P.02/18/2357) and main trial activities are represented. FCM, Ferric Carboxymaltose; iv, intravenous; mth, months; REVAMP-TT, Randomized controlled trial of the Effect of intraVenous iron on Anemia in Malawian Pregnant women - Third Trimester; V0, visit 0; V1, visit 1; V4, visit 4; V7, visit 7; V8, visit 8; V9, visit 9; V10, visit 10; V11, visit 11; V12, visit 12; wks, weeks. Primary outcome measured at V4 or V7, indicated by the red bar connecting both visits. Dotted lines represent follow-up visits not included in the main trial. Figure prepared using BioRender.
| Protocol Activity | Prescreening | Enrolment Week 27–35 | 36 weeks ±2d (pre- delivery) | Delivery ±2d | 28 days postpartum ± 2d | Unscheduled visits |
|---|---|---|---|---|---|---|
| Pre-screening form completion | X | |||||
| Screening | X | |||||
| Informed consent process | X | |||||
| Medical & obstetric history | X | |||||
| Household economic data form | X | |||||
| Household food insecurity | X | |||||
| Complete physical examinationa | X | |||||
| Limited physical examinationb | X | X | X | |||
| Mother Infant Bonding Scale data forms completion | ||||||
| Edinburgh Postpartum depression scale (EPDS) | X | |||||
| Participant arm allocation | X | |||||
| Administer treatment | ||||||
| Intravenous iron | X | |||||
| Oral iron | X | |||||
| Laboratory procedures (Maternal) | ||||||
| Full Blood Count (including Hb) | X | X | X | X | ||
| Hemoglobin (capillary) | X | |||||
| Malaria RDT | X | X | ||||
| Malaria microscopy | X | X | X | X | X | |
| Malaria filter paper for PCR | X | X | X | X | X | |
| Serum for iron markers testsc | X | X | X | X | ||
| Serum for inflammatory markers testsd | X | X | X | X | ||
| Phosphate | X | X | X | X | ||
| Placenta histology | X | |||||
| Breast milk samplef | X | |||||
| Laboratory procedures (Infant) | ||||||
| Full Blood Count | X (cord) | X | ||||
| Malaria microscopy | X (cord) | X | ||||
| Serum for iron markers testsc | X (cord) | X | ||||
| Malaria filter paper for PCR | X (cord) | X | ||||
| Stool samplee | X | |||||
| Pregnancy outcome | X | X | ||||
| Birth weight | X | |||||
| Complete physical examination of babyg | X | |||||
| Limited physical examination of babyh | X | |||||
| Maternal anthropometry: weight, height | X | X | ||||
| Child anthropometry: weight, length, head circumference | X | X | ||||
| Child vaccination and Vit A supplementation status | X | |||||
| Infant neurodevelopment using auditory brainstem responses (ABRs) | X |
aComplete examination: general appearance, throat, neck, thyroid, musculoskeletal, skin, lymph nodes, extremities, pulses, pulmonary, cardiac, abdominal, and neurological examination
bLimited examination: general appearance, brief pulmonary, cardiac, abdominal, and neurological examination
ce.g., Serum ferritin
de.g., CRP
eStool microbiome analysis
fDone only if mothers are still breast feeding
gComplete examination: weight, length, head circumference, APGAR score, Ballard score, new-born adiposity, congenital anomaly, and complications at birth
hLimited examination: general appearance, brief pulmonary, cardiac, abdominal, and neurological examination
REVAMP-TT is a two-arm open-label individual-randomized controlled trial in pregnant women with moderate or severe anemia (capillary Hb<10g/dL) during their third trimester (27-35 weeks gestation). Participants will be randomized to receive either parenteral iron – in the form of FCM– or standard-of-care oral iron. Women are recruited from health centers across Zomba district in Southern Malawi (Figure 1). As REVAMP-TT is an effectiveness trial, the standard-of-care oral iron arm will be provided to participants following the established recommendations issued by the Malawi Ministry of Health. Screening of hemoglobin for eligibility and administration of the intervention (i.e., provision of the intravenous iron) takes place in government health centres. Mothers and their babies will be followed up to 12 months postpartum. The trial was registered with the Australia and New Zealand Clinical Trial Registry (ANZCTR 12621001239853) prior to the start of recruitment and followed the SPIRIT guidelines for randomized trials21 throughout the design and reporting of this protocol.
The trial is based at the Training and Research Unit of Excellence (TRUE) center at Zomba Central Hospital in Southern Malawi. The trial has eight government health centers across Zomba district available for screening, recruitment and provision of the intravenous drug as part of REVAMP-TT, namely: Likangala, Bimbi, Lambulira, Domasi, Naisi, Matawale, Sadzi and City clinic (Figure 1). These facilities receive close to 1500 antenatal visits per month. All subsequent study visits will be done at TRUE center in Zomba.
Participants are eligible for the trial if they have a confirmed singleton pregnancy 27- to 35-weeks’ gestation either using last menstrual period or fundal height, have a capillary Hb concentration of less than 10 g/dL, measured by HemoCue 301+, and have negative malaria RDT. Additionally, the participants must be afebrile with no evidence of septicemia, reside and plan to give birth within the study catchment area of Zomba district and be able to provide written informed consent (or assent if <18 years old).
Women are excluded if they were previously enrolled in the REVAMP trial16,18 or are actively participating in another intervention trial. Women with known hypersensitivity to any of the study drugs, clinical symptoms of malaria or other infection, any condition requiring hospitalization, known history of sickle cell or sickle-hemoglobin C anemia, clinically unstable with a low hemoglobin level requiring a blood transfusion (usually Hb <5g/dL), or pre-eclampsia are excluded from participating in this trial. HIV positivity is not an exclusion.
Using a randomization schedule of randomly permuted blocks stratified by site to achieve balance between the arms within each site, participants are randomly allocated 1:1 to one of the two treatment arms. An independent statistician computer-generated the randomization list, and sealed, opaque envelopes were used to randomly assign participants. Despite the trial being open-label, laboratory scientists measuring hemoglobin concentration, midwives collecting birth outcome data, and investigators and researchers at the Walter and Eliza Hall Institute, Melbourne, Australia (including data managers and statisticians) are all blinded to the treatment allocation throughout the trial duration until the database is locked and ready for unblinding.
This trial takes place in primary health centers spread across Zomba district in Malawi, with several of the health centers considered to be remote – Likangala, Bimbi and Lambulira. (Figure 1). As REVAMP-TT is delivered through government health centers using the available government health centers’ infrastructure, we used a co-design approach to develop interventions to support its implementation (REVAMP-IS study17). Co-design is a two-staged approach to health systems and service improvements that typically involves an information-gathering stage followed by a second stage in which end users jointly develop the strategies with health service providers and the project leaders22. Where the workload permits, government nurses directly administer the intervention and, in all clinics, government nurses received training and are encouraged to assist the trial team. Women in the intervention groups receive intravenous iron in the form of ferric carboxymaltose (Vifor Pharma) at a dosage of 20 mg/Kg body weight if <50 kg and a maximum of 1000 mg if body weight ≥50 kg given over 15 minutes in 250 mL of normal saline, once on recruitment day. The participant is observed for a minimum of 30 minutes before being discharged home. Women in the control group receive oral iron-200 mg ferrous sulphate (approx. 65 mg elemental iron) twice daily for the remainder of pregnancy. Importantly, the oral iron is given under real-life health service delivery conditions where the participant is given three months of oral iron at presentation to the antenatal clinic. This strategy is being employed as it is a component of the key hypothesis of this trial that there is a reduced effect of oral iron on maternal anemia due to poor adherence to the full course of treatment. In addition, all participants receive IPTp with SP, 1500 mg sulfadoxine and 75 mg pyrimethamine (three fixed tablets of SP strength at 500 mg/25 mg) as recommended in the national guidelines during study follow up visits23. As part of the safety assessment, IPTp-SP post-randomization is directly observed by study staff.
All study activities at each study visit are summarized in Table 1.
Data are recorded in digital form with REDCap using electronic tablets and backed up nightly to a local backup server at TRUE, Blantyre, Malawi, with a de-identified fortnightly backup to the servers at the Walter and Eliza Hall Institute, Melbourne, Australia. REDCap is hosted on infrastructure belonging to the trial’s organizational team in Malawi and is subject to the same security and backup regimen as other systems (e.g., the network file servers).
The primary outcome is the proportion of women with anemia (defined as a venous blood hemoglobin < 11 g/dL) at 36 weeks gestation or delivery, whichever comes first. Gestational age in this setting is evaluated by last menstrual date and/or measurement of fundal height. These were chosen due to their common use in the government primary care centers where there is no availability of ultrasound, thus ensuring the generalizability of the results.
Maternal secondary outcomes include hemoglobin, ferritin, anemia, iron deficiency, iron-deficiency anemia collected at 36 weeks gestation, delivery, and at one-month postpartum. Iron-deficiency is determined by measuring levels of serum ferritin and setting thresholds at ferritin<15ug/L or ferritin<30ug/L if C-reactive protein>5mg/L. Iron deficiency anemia indicates Hb<11g/dL and serum ferritin<15ug/L or ferritin<30ug/L if C-reactive protein>5mg/L24.
Neonatal outcomes include birth weight and low birth weight (birth weight <2,500g) within 24 hours of delivery and infant length-for-age z-score, weight-for-age z-score, and weight-for-length z-score as well as iron and hematological parameters collected at one-month postpartum.
Safety outcomes for this trial include maternal and infant adverse events and severe adverse effects, hypophosphatemia (defined as Phosphate (PO4 <0.80 mmol/L), and inflammation status (threshold for inflammation set at C-reactive protein>5mg/L), measured at the same timepoints as specified for primary and secondary outcomes detailed above.
The sample size for the trial was to recruit 590 pregnant women (295 per arm) to have 90% power to detect that FCM will result in a 14% reduction in absolute anemia prevalence compared with standard-of-care oral iron (49% to 63%)25, allowing for a two-sided alpha of 5% and a 10% loss to follow up at the primary outcome. The sample size also has 72% to 97% power to detect an absolute difference between standard-of-care oral iron and FCM of 100 to 150g in the neonatal outcome of birth weight, assuming a standard deviation of 450g7 and a two-sided alpha of 5%, after accounting for a miscarriage and stillbirth rate of 1%. Sample size calculations were performed using Stata/SE (StataCorp. 2019. College Station, TX: StataCorp LLC). The open-access software R can be used for sample size calculations where Stata/SE is unavailable.
The design incorporated an adaptive sample size re-estimation procedure based on the “promising zone” methodology of Mehta and Pocock26 when the primary maternal outcome of at least 50% of the recruited participants were obtained. A sample size increase by up to 260 participants to the pre-specified maximum of 850 participants total (including loss to follow up) was allowed with the aim to achieve a conditional power of 90%, if the conditional power was in the “promising zone”. Using a one-tailed alpha of 0.025 and a power of 90%, the “promising zone” for conditional power was 0.388 to 0.9. The pre-planned sample size of 590 participants was confirmed as the final sample size after execution or the method by an independent unblinded statistician using the data from 77.8% (459/590) of the participants and recommendation from the Data Monitoring Committee. Sample size re-estimation was performed using a user-written program (Excel version 16.71).
An independent Data Monitoring Committee (DMC) oversees the trial and is comprised of three international experts in clinical trials, obstetrics, epidemiology and statistics. The DMC recommends to the sponsor and investigators whether to continue, modify or terminate the trial on ethical grounds. The DMC evaluated the results of the sample size re-estimation (see sample size estimation and power calculation) and recommended that no sample size change was necessary.
Efficacy analyses will be performed according to the participants randomized treatment group and safety analyses will be performed according to the participants actual treatment group. Frequencies and percentages will be reported for categorical variables and mean and standard deviation or median and quartiles (25th and 75th percentile) for continuous variables. Treatment effects will be presented as point estimates with two-sided 95% confidence intervals (CIs). A two-sided P value <0.05 will be used to indicate statistical significance for the primary outcome and birth weight. The Holm procedure27 will be used to control the family-wise error rate at 0.05 for maternal outcomes (hemoglobin concentration, and serum ferritin concentration at 36 weeks gestation or delivery (whichever comes first) and at one-month postpartum) and neonatal outcomes (hemoglobin concentration, serum ferritin concentration and weight at one-month of age) separately. Specifically, the Holm procedure is a stepwise multiple test method that controls the probability of one or more type 1 errors to be at most 5%, whereby null hypotheses are tested step-down using adjusted individual significance levels until no further rejections can be made26. Analyses will be conducted using Stata/SE (StataCorp. 2019. College Station, TX: StataCorp LLC). The open-access software R can be used for sample size calculations where Stata/SE is unavailable.
A log-binomial regression model will be used to examine maternal anemia at the primary timepoint – 36 weeks’ gestation or delivery, whichever comes first – with study participants as a random intercept to account for multiple timepoints. A treatment and treatment-by-study-visit interaction and an adjustment for the stratification variable site (used during randomization) will be included in the model. The reference group for the model will be the standard-of-care oral iron treatment group. The prevalence ratio of IV iron versus standard-of-care oral iron will be estimated from this model as the treatment effect. In the event of non-convergence, we will use alternative models.
Identical to the analysis of the primary outcome repeated time-point binary outcomes (anemia, moderate/severe anemia, iron deficiency, iron deficiency anemia) will be analyzed. A likelihood-based longitudinal data analysis model28 will be used to examine secondary repeated time-point continuous outcomes (hemoglobin concentration and ferritin concentration). Unstructured variance-covariance among the repeated measurements and a common baseline mean will be assumed by the model for the two treatment arms. The main effects of the model will be the stratification factor (site), the treatment and treatment by study visit interaction, and the study visit (timepoint) as a categorical variable. We will consider alternative structures (first-order autoregressive, Toeplitz, compound symmetry) in the event of non-convergence. The mean change between IV iron and standard-of-care oral iron will be used to estimate the treatment effect using this model. Before analysis, ferritin (μg/L) will be loge transformed, and the treatment effect will be expressed as a geometric mean ratio. A linear regression model, adjusting for the stratification factor (site) will be used to analyze birth weight. The absolute difference in mean birth weight between IV iron and standard-of-care oral iron will be used to estimate the treatment effect based on this model.
Using the same analysis as used for birth weight, birth length within 24 hours of birth and hemoglobin concentration, ferritin concentration, length-for-age z-score, weight-for-age z-score, and weight-for-length z-score at one-month of age will be analyzed. If the variables are considered skewed, appropriate transformations may be applied to them prior to fitting the model. A log-binomial regression model, adjusting for the stratification factor (site), will be used to examine low birth weight and stillbirth. The risk ratio of IV iron versus standard-of-care oral iron will be estimated from this model as the treatment effect. In the event of non-convergence, we will use alternative models.
Analyses of maternal and neonate adverse event outcomes, and neonate safety biomarkers (hypophosphatemia and malaria RDT positive) will be analyzed similarly to analyses of secondary single time-point binary outcomes detailed above. Maternal safety biomarkers (hypophosphatemia, inflammation and malaria RDT positive) will be analyzed using the same analysis as the primary maternal outcome (anemia).
Using a model similar to the main analysis model of the primary outcome described above, the treatment effect at 36 weeks’ gestation will be examined for the primary outcome. Adjusted analyses for pre-specified covariates and analysis excluding participants with protocol violations (e.g., multiple births) will be included in additional analyses of primary, key secondary and other secondary outcomes. To determine the heterogeneity of the differences between IV iron and standard-of-care oral iron six pre-specified subgroup analyses (gravidity (primigravida vs. multigravida), baseline HIV status (positive vs negative), baseline severe anaemia status (yes vs no severe anaemia), baseline iron-deficient status (yes vs no ID), baseline iron-deficient anaemia status (yes vs no IDA), baseline inflammation status (yes vs no elevated CRP)) will be performed for primary and key secondary maternal and neonate outcomes. The missing-at-random assumption will be used to fit the model for the primary maternal outcome (anemia) and the missing-completely-at-random assumption will be used to fit the model for the primary neonate outcome (birth weight). Pattern-mixture models will be conducted to assess sensitivity of the results to these assumptions.
The trial started recruiting participants on the 24th of November 2021 and completed enrolment on the 22nd of February 2023. Follow-up visits for all participants were concluded in June 2023 for one-month postpartum and are expected to continue until May 2024, for one year postpartum.
This protocol received the approval of the National Health Sciences Research Committee of Malawi Approval - NHSRC REF. Number: 20/11/2622, the Human Research Ethics committee at WEHI – HRE REF. Number: 20/25, and the Malawi Pharmacy and Medicines Regulatory Authority –PMRA/CTRC/III/08062021130.
Informed consent will be obtained from each participant before conducting any study related procedure. The information will be provided in the local language (Chichewa) of the participant and the participant will be given the opportunity to ask questions and allowed time to consider the information provided. If the participant is unable to write their signature, then a thumbprint may be used. If the participant is unable to read the information her/himself, full and comprehensive information will be communicated to the participant in the presence of a witness. Each original signed informed consent will be kept on file.
At the end of the study, the results will first be disseminated to national policymakers, government departments, academics from local research institutions and universities, National Health Sciences Research Committee, the College of Medicine Research and Ethics Committee and professional bodies in Malawi at the national stakeholders’ meeting or research dissemination conferences to be held in the country.
Research results will also be disseminated to the global research community, technical agencies, and international government bodies via peer-reviewed journals and at international scientific fora.
Study data will be made accessible under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0), comprising underlying de-identified individual participant data relevant to the reported trial results and a data dictionary.
The high prevalence of anemia during pregnancy in Africa, and specifically in Malawi, continues to be a major public health concern. This trial will provide the necessary evidence of the effectiveness of implementing intravenous iron for the treatment of anemia during pregnancy in the third trimester. The risk of adverse pregnancy outcomes due to anemia is particularly high in the third trimester, which is also the period when most pregnant women in LMICs present themselves for antenatal check-up. While IV iron has been used extensively in developed countries, this trial represents the first attempt to bring this intervention to primary health centers in LMICs. This trial will help determine whether Ferric Carboxymaltose represents an effective, safe, and feasible approach for the treatment of moderate to severe anemia in the third trimester of pregnancy, particularly in resource-limited countries such as Malawi.
If the trial demonstrates the effectiveness and safety of FCM in the third trimester, it could lead to a paradigm shift in the management of anemia during pregnancy in LMICs. By providing evidence-based guidance on the use of IV iron, this trial has the potential to improve maternal and fetal outcomes, reduce the risk of postpartum haemorrhage, and enhance infant health. Finally, the results of this trial will be useful in guiding policy decisions related to the use of IV iron in resource-limited settings, ultimately improving the overall health of pregnant women and their offspring in Malawi and other African countries.
We would like to acknowledge the study participants as well as the dedication of the research staff in Zomba, Malawi and Melbourne, Australia. We are grateful for the support we continue to receive from the District Health Offices in Zomba district of Malawi as well as the managerial and nursing staff of the government health centres where the trial is conducted. We acknowledge the collaboration with the Zomba Central Hospital, especially the office of the Hospital Director. We acknowledge the effort of the labour ward nurses and staff. We also acknowledge Dr Kamiza’s Histopathology Laboratory in Blantyre, Malawi, for their analysis of the placental samples.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Haematology, pregnancy, iron management
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Haematology, pregnancy, iron management
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Iron deficiency anemia, pregnancy, health services research
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 18 Dec 23 |
read | |
|
Version 1 05 Sep 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)