Keywords
Population-based, Households, Serosurvey, Serology, SARS-CoV-2, COVID-19, Rural, urban informal settlement, transmission, seroprevalence, Kenya
Population-based, Households, Serosurvey, Serology, SARS-CoV-2, COVID-19, Rural, urban informal settlement, transmission, seroprevalence, Kenya
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the resulting disease, coronavirus disease (COVID-19) was declared a public health emergency of international concern (PHEIC) by the World Health Organization (WHO) on 30 January 20201,2. By the first week of January 2022, close to 300 million confirmed cases and over 5.4 million deaths had been reported worldwide3. In Kenya, since 13th March 2020, when the first case of SARS-CoV-2 infection was reported, a total of 295,098 cases and 5,378 deaths (case fatality rate of 1.8%) had been reported by the Ministry of Health (MoH) as of 31st December 20214. In response to the pandemic, the Kenyan MoH employed various strategies, including advocating for the 3Ws (for instance, wearing of masks, washing your hands, watching distance), restriction of movement, and later administration of COVID-19 vaccine to reduce the risk of infection and severe disease.
SARS-CoV-2 has extensively spread in cities and rural communities. Most infected persons generate detectable antibodies that persist for up to a year. Leveraging existing surveillance programs for COVID-19 surveillance and for seroprevalence surveys offer an opportunity to accelerate the monitoring of the extent of transmission of SARS-CoV-2 infections in populations. Population-based surveillance platforms have especially been singled out as integral in generating data to show the temporal and spatial spread of the SARS-CoV-2 virus; as well as evaluate the impact of non-pharmaceutical and pharmaceutical interventions5. Furthermore, systematic longitudinal surveillance in these platforms provides the much-needed information on the magnitude of exposure in the population and the duration of immune responses among the infected individuals. These data are useful in informing the MoH and other agencies on the magnitude of population exposure to SARS-CoV-2 infection and in influencing the design and delivery of public health mitigation measures.
To inform the COVID-19 response in Kenya, active (household-based serial longitudinal serosurveys) and passive (health facility screening for patients presenting with respiratory symptoms) surveillance approaches were activated to track the extent of the spread of SARS-CoV-2 infections in two well-defined populations in an urban, densely populated, informal settlement in Nairobi and a rural setting in western Kenya6,7. We present findings from the longitudinal household-based serial seroprevalence surveys with a detailed context of SARS-CoV-2 circulation in these populations. The seroprevalence surveys were aligned with the UNITY seroepidemiological protocol by WHO8 and followed a serosurvey performed in December 2020 in Kibera, Nairobi9. Describing the SARS-CoV-2 transmission waves against the temporal patterns of population-level seroprevalence further provides much needed data to inform the review, revision, and implementation of context-appropriate mitigation strategies, including the deployment of vaccines.
The study leveraged on an ongoing population-based infectious disease surveillance (PBIDS) platform. The PBIDS platform conducts surveillance within two well-characterized populations in Asembo (rural western Kenya in Siaya County) and in Kibera (the largest urban, densely populated, informal settlement in Nairobi) and is run by Kenya Medical Research Institute-Centre for Global Health Research (KEMRI-CGHR) with technical and financial support from US Centers for Disease Control and prevention (CDC)6,7. The platform has been running since 2006 and the surveillance objectives and methods have been described previously6,7. Briefly, the PBIDS platform aims to monitor burden and aetiology of common and (re)emerging acute infectious diseases and evaluate the impact of public health interventions in the two populations in Kenya, Figure 1. In Kibera, PBIDS covers two villages characterized by very poor water purification, supply, and waste disposal, and a large number of infectious diseases including an adult HIV prevalence of 15%6,10. The surveillance area covers 0.40 km2, with a high population density (~77,000 individuals/km2). The Asembo site covers 33 villages that are sparsely populated (~350 individuals/km2) in ~80 km2. The area is culturally homogeneous (95% Luo ethnicity); subsistence farming and fishing constitute the principal economy. The area has perennial, high-level malaria transmission and has reported high prevalence of HIV (~15%) among adults aged 15–64 years. Consequently, both sites have mortality rates that reflect a high number of infectious diseases11–13.
Maps data: Google, ©2022.
Enrolled households are followed regularly (once in 2020 due to COVID-19 interruptions from April to September and thrice in 2021) to collect data on recent illnesses, healthcare-seeking behaviour, and demographic characteristics. The households are within 5 km radius of St Elizabeth Lwak Mission Hospital (LMH) in Asembo and a 1 km radius of the Tabitha Medical Clinic in Kibera. The active PBIDS participants receive free medical care for acute illnesses at the centrally located surveillance clinics. All households within the defined radius are invited to participate in PBIDS and enrolment is continuous. As of December 2020, the Asembo PBIDS had 34,999 persons in 9,225 households, while Kibera PBIDS had 23,103 persons in 5,265 households under follow-up.
A longitudinal cohort of randomly selected households from the PBIDS database were enrolled and serial sampling of all available household members conducted in March and June 2021, Figure 2. The target was to enrol approximately 900 individuals in each site. For unavailable households and household members, three attempts for enrolment were made before randomly selected replacement households were considered. The survey size was anticipated to detect a seroprevalence of 45% with a precision of 5% and design effect of 2 at 95% confidence interval. An attrition of 20% was also incorporated. Our expected seroprevalence of 45% was informed by earlier serosurveys reporting a seroprevalence of 13% among blood bank specimens in Kenya14,15 and an observation of 3.6 times higher seroprevalence among informal settlement residents compared to non-informal settlement residents in India16.
The household members’ consenting, enrolment, data and blood collection were conducted through home visits by trained field teams consisting of a field worker and a phlebotomist. Venous blood samples (approximately 5 ml for persons aged >12 years; 2–3 ml for children 2–12 years and 1.5 ml for children <2 years) were collected from each participant and transported in a cool box at 2-8°C to the laboratory on the same day. Sera were separated from the whole blood specimen and stored at -80oC before testing.
At the surveillance clinic in each site, PBIDS participants of all ages presenting with severe acute respiratory illness (SARI) were consented for a nasopharyngeal and oropharyngeal (NP/OP) specimen. Since 1st May 2020, all the NP/OP specimens collected were tested for SARS-CoV-2 using real time Reverse Transcription-Polymerase Chain Reaction (rRT-PCR). From September 2020, the eligibility criterion for NP/OP collection was expanded to include any patients presenting with acute febrile illness (AFI) or acute respiratory illness (ARI) as well as known contacts of confirmed COVID-19 cases regardless of their symptom status. Table 1 presents the case definitions used in the enhanced COVID-19 surveillance.
| Case1 | Definition |
|---|---|
| SARI | For <5 years: patients presenting with cough or difficulty breathing, plus chest indrawing or oxygen saturation <90% or WHO danger signs (inability to feed, impaired consciousness, vomiting). For >=5 years: patients presenting with cough or difficulty breathing or chest pain, plus temperature >=38.0°C or oxygen saturation <90%. |
| AFI | Patients presenting with temperature of >=38.0°C or recent history of fever. |
| ARI | Patients presenting with cough or difficulty in breathing or running/blocked nose, or sore throat. |
| COVID-19 contacts | Patients reporting close contacts with confirmed COVID-19 cases regardless of their symptom status. |
Key: 1, SARI was used for COVID-19 surveillance from May 1st, 2020 and the rest added from September 1st, 2020 onwards. PBIDS, population based infectious disease surveillance; COVID-19, coronavirus disease; SARI, Severe acute respiratory illness; AFI, Acute febrile illness; ARI, Acute respiratory illness; WHO, World Health Organization.
All laboratory tests were performed in an international organization for standardization (ISO)15189 certified and Good Clinical Practice-accredited CDC-supported laboratories at KEMRI-CGHR in Kisumu and Nairobi, Kenya. The serum specimens were tested for anti- Spike IgG antibodies according to manufacturer’s instructions using the SCoV-2 Detect™ IgG ELISA kit (Catalogue #64824, InBios International, Inc, USA). The kit manufacturer reports of 92% and Specificty of 99%17,18. The NP/OP swabs were tested by rRT-PCR for SARS-CoV-2 and select positives sequenced for variant detection using partial and full genome sequencing at the CDC-supported laboratories in Kisumu.
All data cleaning, management and analyses were performed using Stata 15.1 software (STATA Corp, Texas, USA) (free alternative, RStudio) in accordance with methods we previously published9. Briefly, individual seroprevalence of SARS-CoV-2 antibodies was defined as a percentage of the seropositive individuals among those tested accounting for household clustering and weighting by age and sex distribution of the PBIDS general population in each surveillance site as of March 2021 (Figure 3). The standard errors for generating the 95% confidence intervals were computed using the Taylor linearized variance estimation method19. Pearson’s chi-squared test was used to assess the association of categorical variables with individual seropositivity. Household seroprevalence (defined as the percentage of households with at least one seropositive member) was estimated and stratified by household size (usual number of persons in the household), and number of persons enrolled in the serosurvey per household. Age, sex, relationship to head of the household, main occupation, household size, and underlying medical conditions associated with serious COVID-19 complications (e.g., known hypertensive, asthmatic or diabetic) were considered in the univariable logistic regression model for determining the factors associated with individual seropositivity for each site and survey round. Age and sex were considered a priori for inclusion in the multivariable logistic regression. The final multivariable logistic regression model also included variables with p-value of ≤0.05 and accounted for sampling weights and clustering by household using the clustered sandwich estimator20,21. Adjusted odds ratio (aOR) and 95% confidence intervals (CI) were presented and two-sided p-values <0.05 were considered statistically significant. In addition, weekly SARS-CoV-2 positivity (percentage of RT-PCR positive cases from the total tested in a week) by rRT-PCR from the health facility surveillance is presented to provide context to the seroprevalence surveys up to 31st December 2021.
Individual written informed consent/verbal assent was obtained from all the study participants and/or their parents/guardian. Ethical approval for the study was provided by the KEMRI Scientific and Ethical Review Committee in Kenya (#4168, February 15th, 2021). This activity was also reviewed by CDC and was conducted consistent with applicable federal law and CDC policy as provided for in the Code of Federal Regulations (45 C.F.R part 46 and 21 C.F.R. part 56). The PBIDS protocol is approved by KEMRI Scientific and Ethical Review Committee in Kenya (#2761), Washington State University reliance agreement and CDC institutional review board (#6775).
From 1st May 2020 to 31st December 2021, SARS-CoV-2 was detected at varying intensities at different time periods, as shown in Figure 4. In Asembo, three waves were observed by 31st December 2021: first wave in September to December 2020, second wave from March to July 2021 and the last from early December 2021 and ongoing by the end of the reporting period. In comparison, Kibera had five waves: first wave was ongoing from the beginning of the COVID-19 surveillance in May 2020 and ended in August 2020, second wave from September 2020 to January 2021, third wave from February 2021 to May 2021, fourth from June to October 2021, and fifth wave began in November 2021 and was ongoing by the end of the reporting period. The second wave in Asembo and the fourth in Kibera were predominated by the Delta variant (unpublished reports, Clayton Onyango) and started earlier in Asembo in western Kenya before being detected in Kibera in Nairobi. The latest wave was associated with Omicron (BA.1) variant (unpublished reports, Clayton Onyango) and had the highest weekly RT-PCR positivity at the peak in December 2021 of 50% in Asembo and 87% in Kibera.
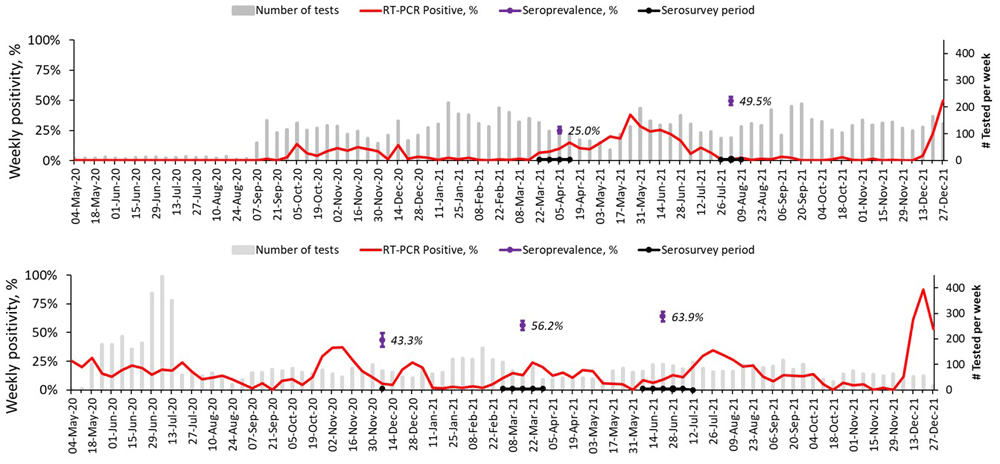
Included are the overall weighted individual seropositivity in the respective times of the serosurvey in Asembo and Kibera.
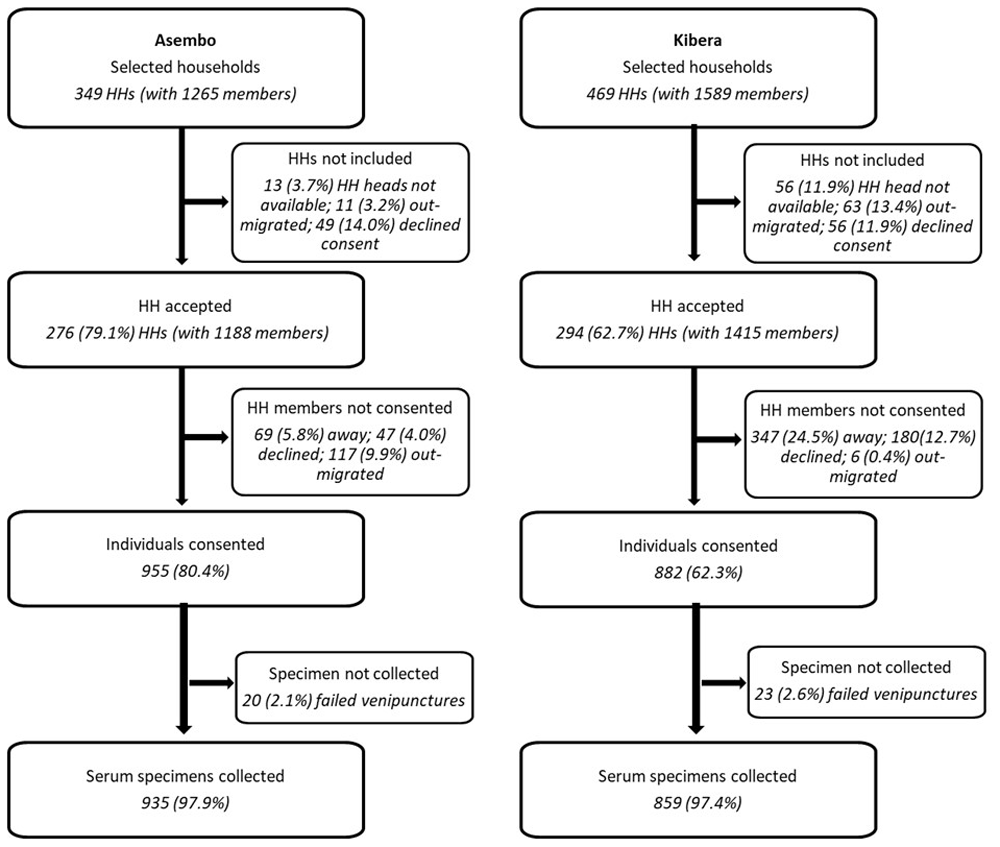
Of the 349 randomly selected households (with 1,265 members) in Asembo, household heads in 79.1% (276 households with 1,188 eligible members) gave permission for their households to participate (Figure 2). Of the 469 randomly selected households (with 1,589 members), household heads in 64.6% (294 households with 1,415 eligible members) agreed to participate in Kibera. Of the 76 households in Asembo that did not participate, 37 (48.7%) declined participation, 13 (17.1%) did not have a household head available for consenting, 11 (14.5%) had moved, and 12 (15.8%) provided no reason for not participating. In Kibera, 172 households did not accept to participate; 44 (25.6%) declined participation, 56 (32.6%) did not have a household head available for consenting, 63 (36.6%) had moved, and 12 (0.6%) provided no reason. Individual written informed consent was obtained from 955 (67.5% of eligible members) and 882 (74.2%) participants in Asembo and Kibera, respectively. Of the 233 who didn’t consent in Asembo, 115 (49.4%) had out-migrated, 69 (29.6%) were not found at home, even after three attempts, 47 (20.2%) declined and two (0.9%) provided no reason. In Kibera, 533 did not consent; 347 (65.1%) were not found at home, 145 (27.2%) declined participation, 6 (1.1%) had out-migrated and 35 (6.6%) provided no reason.
The median number of individuals in a household was three (interquartile range (IQR), 1–5; range, 1–15) in Asembo, and three (IQR, 1–5; range, 1–19) in Kibera while the corresponding median number of consented individuals per household was three (IQR, 2–5; range, 1–13) in Asembo and three (IQR, 1–4; range, 1–11) in Kibera.
In Asembo, female participants aged 50 years and above, male participants over 60 years and both sexes aged 5–19 years old were overrepresented, while men aged 20–29 years were underrepresented in the two surveys, relative to the PBIDS population (Figure 3). For Kibera, male children aged 5–9 years and women aged 30 years and above were overrepresented, while both female and male children below 5 years of age and men aged 30–59 years were underrepresented in both surveys, compared to the PBIDS general population (Figure 3).
The first serosurvey (round one, R1) was conducted between 19th February and 28th March 2021 (median date, 11th March 2021), and the second round (R2) between 4th June to 4th July 2021 (median date, 15th June 2021) yielding 859 and 750 serum specimens, respectively, in Kibera (Table 2). In Asembo, 935 and 888 sera specimens were collected in the first (12th March to 8th April 2021, median date, 25th March 2021) and second (8th July to 4th August 2021, median date 19th July 2021) serosurvey, respectively. The median number of sampled individuals per household was three (IQR, 2–5; range, 1–11) in Asembo and three (IQR, 1–4; range, 1–11) in Kibera. During the second round of serosurveys, consent was obtained for an additional 115 and 160 individuals and they were sampled from the participating households in Asembo and Kibera, respectively. These were mainly individuals who were not available in the households for enrolment during the first round of the serosurvey.
The majority of the sampled individuals during the two rounds in both sites were female (55.4% in Asembo and 57.5% in Kibera in the first round, Table 2). The average age in years for participants was higher for Asembo participants compared to Kibera participants (26.6 (95% CI: 25.3, 28.0) versus 22.7 (95% CI: 21.7, 23.8) years during the first serosurvey).
Of the tested individuals, 234 (25.0%) and 440 (49.5%) were seropositive in Asembo for the first and second serosurvey, respectively. Correspondingly, 478 (55.6%) and 483 (64.4%) individuals were seropositive in the respective rounds in Kibera. The overall weighted individual seroprevalence increased from 56.2% (95% CI: 52.1, 60.2%) in March 2021 to 63.9% (95% CI: 59.5, 68.0%) in June 2021 in Kibera. For Asembo, the weighted seroprevalence almost doubled from 26.0% (95% CI: 22.4, 30.0%) in March 2021 to 48.7% (95% CI: 44.3, 53.2%) in July 2021. At the respective time points for the serosurvey, Kibera had consistently higher seroprevalence than Asembo. In both sites and for each of the two rounds, the seroprevalence did not vary by sex, but was lower in children compared to adults (Table 3). An appreciable increase in age-specific individual seroprevalence over the rounds was observed in all age groups in Asembo (14.3–28.5% increase) and among young participants in the 5–19 years age groups (11.8–17.7%) in Kibera.
In Kibera, the age effect was also observed for seroprevalence estimates by relationship to the household head in the first round with grandchildren (25.0%; 95% CI: 10.1, 51.2%) and children (52.1%; 95% CI: 47.1, 57.1%) registering lower estimates compared to the household head (59.6%; 95% CI: 51.1, 67.7 %) and other adults including spouses (70.9%; 95% CI: 62.9, 77.7%) and other relatives (54.1%; 95% CI: 30.1, 76.4%). However, these differences in seroprevalence by relationships were not statistically significant in the second serosurvey in Kibera as well as in both rounds in Asembo.
Seroprevalence in each round and site increased with the level of education, with the lowest among those with no education and highest among those with secondary or above level of education—again highlighting the age effect as the young children were coded to have no education. There was no obvious pattern in seroprevalence based on main occupation groups (Table 3) for the two sites and over the two survey rounds. Participants with any underlying medical conditions had similar seroprevalence as those without in each round and site (Table 3).
COVID-19 vaccine uptake was low at the time these serosurveys were conducted. None of the study participants had received the COVID-19 vaccine before round one of the serosurvey in both sites. By round two, 11 (1.2%) participants reported having received the COVID-19 vaccine in Asembo, while in Kibera, 12 (1.6%) reported receipt of the vaccine—only one participant in Asembo and three participants in Kibera reported receipt of two doses. All the vaccinated participants had received Oxford-AstraZeneca vaccine except for one participant from Kibera who reported receiving Johnson & Johnson COVID-19 vaccine. Of the 23 individuals who reported having received the COVID-19 vaccine in round two of the serosurvey, 18 (78.3%) were seropositive—nine from each site. Among those reporting receipt of COVID-19 vaccine, seropositivity was 81.8% in Asembo and 75.0% in Kibera.
Samples tested were from 276 and 262 households in Asembo and 294 and 252 in Kibera for round one and two, respectively (Table 4). Among sampled households in Asembo, 145 (52.5%; 95% CI: 46.5–58.6%) and 208 (79.4%; 95% CI: 74.0–84.1%) had at least one seropositive participant in the first and second surveys, respectively. In Kibera, 237 households (80.6%; 95% CI: 75.6–85.0%) and 220 households (87.3%; 95% CI: 82.5–91.1%) had at least one seropositive participant in round one and two, respectively. Within households with at least one seropositive individual, the median (IQR) percentage of seropositive individuals in the household was 40.0% (25.0–60.0%) and 63.3% (40.0–100%) in Asembo for round one and two, respectively. The corresponding median (IQR) estimates for Kibera were higher at 75.0% (50.0–100%) and 80.0% (50.0–100%). Of 223 and 210 households with two or more members enrolled in Asembo, 133 (59.6%) and 176 (83.8%) had at least one person seropositive in the respective survey rounds. On the other hand, the mean seropositivity among households with at least one seropositive person and two or more participants enrolled in Asembo was 42.3% (95% CI: 38.4–46.1%) and 58.6% (95% CI: 54.7–62.6%) in round one and two of the serosurvey, respectively. For Kibera, the corresponding mean seropositivity in the similar households was 62.7% (95% CI: 58.9– 66.6%) and 68.1% (95% CI: 64.3–71.9%) in round one and two of the serosurvey, respectively.
| Characteristics | Categories | Round | Asembo | Kibera | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | n2 | HH P3, % | 95% CI | N | n | HH P, % | 95% CI | |||||
| Overall | All | 1 | 276 | 145 | 52.5 | 46.5 | 58.6 | 294 | 237 | 80.6 | 75.6 | 85.0 |
| 2 | 262 | 208 | 79.4 | 74.0 | 84.1 | 252 | 220 | 87.3 | 82.5 | 91.1 | ||
| Household size | 1 | 1 | 35 | 7 | 20.0 | 8.4 | 36.9 | 26 | 18 | 69.2 | 48.2 | 85.7 |
| 2 | 41 | 24 | 58.5 | 42.1 | 73.7 | 38 | 25 | 65.8 | 48.6 | 80.4 | ||
| 2 | 1 | 42 | 19 | 45.2 | 29.8 | 61.3 | 21 | 14 | 66.7 | 43.0 | 85.4 | |
| 2 | 44 | 28 | 63.6 | 47.8 | 77.6 | 30 | 26 | 86.7 | 69.3 | 96.2 | ||
| 3 | 1 | 44 | 22 | 50.0 | 34.6 | 65.4 | 36 | 25 | 69.4 | 51.9 | 83.7 | |
| 2 | 43 | 35 | 81.4 | 66.6 | 91.6 | 54 | 44 | 81.5 | 68.6 | 90.7 | ||
| 4 | 1 | 40 | 18 | 45.0 | 29.3 | 61.5 | 57 | 48 | 84.2 | 72.1 | 92.5 | |
| 2 | 37 | 31 | 83.8 | 68.0 | 93.8 | 61 | 58 | 95.1 | 86.3 | 99.0 | ||
| 5 | 1 | 40 | 31 | 77.5 | 61.5 | 89.2 | 49 | 43 | 87.8 | 75.2 | 95.4 | |
| 2 | 45 | 43 | 95.6 | 84.9 | 99.5 | 33 | 31 | 93.9 | 79.8 | 99.3 | ||
| 6 | 1 | 75 | 48 | 64.0 | 52.1 | 74.8 | 105 | 89 | 84.8 | 76.4 | 91.0 | |
| 2 | 52 | 47 | 90.4 | 79.0 | 96.8 | 36 | 36 | 100.0 | 90.3 | 100.0 | ||
| Household size, grouped | 1-2 | 1 | 77 | 26 | 33.8 | 23.4 | 45.4 | 47 | 32 | 68.1 | 52.9 | 80.9 |
| 2 | 85 | 52 | 61.2 | 50.0 | 71.6 | 68 | 51 | 75.0 | 63.0 | 84.7 | ||
| 3-4 | 1 | 84 | 40 | 47.6 | 36.6 | 58.8 | 93 | 73 | 78.5 | 68.8 | 86.3 | |
| 2 | 80 | 66 | 82.5 | 72.4 | 90.1 | 115 | 102 | 88.7 | 81.4 | 93.8 | ||
| ≥5 | 1 | 115 | 79 | 68.7 | 59.4 | 77.0 | 154 | 132 | 85.7 | 79.2 | 90.8 | |
| 2 | 97 | 90 | 92.8 | 85.7 | 97.0 | 69 | 67 | 97.1 | 89.9 | 99.6 | ||
Age was a predictor of individual seropositivity. Relative to adults aged 20–29 years, young age groups (<20 years) had lower odds of being seropositive in the respective sites and rounds. The odds of being seropositive were similar for older adults (age groups ≥30 years) compared to the reference group, 20–29 years. The odds of being seropositive among the elderly groups (≥60 years) were not different from the referent 20–29 years age group across the sites and by survey round. Relative to household heads, spouses had higher odds of being seropositive in Asembo round two (adjusted Odds Ratio, aOR, 2.47; 95% CI: 1.35–4.54) and Kibera round one (aOR; 1.89 (95% CI: 1.08–3.31). During round two of serosurvey in Asembo, the odds of seropositivity were higher in the employed participants compared to those not employed, aOR, 3.37 (95% CI: 1.76–6.45). This association of employment status and seropositivity was not observed in Kibera. Sex and highest level of education were not significantly associated with individual seropositivity for any of the sites or rounds (Table 5).
Of the individuals enrolled in round one, 754 (80.6%) in Asembo and 606 (70.6%) in Kibera participated in the second serosurvey. The median duration between the serosurveys was longer in Asembo (3.9 months; IQR, 3.6–4.0 months) than in Kibera (3.2 months; IQR, 2.9–3.4) months). Between the two serosurveys, 215/564 (38.1%) of the previously seronegative participants seroconverted in Asembo (median age, 18.1 (IQR, 11.8–40.1 years) and 98/273 (35.9%) in Kibera (median age, 12.1 (IQR, 6.7–25.1 years). Conversely, 29/190 (15.3%) of the previously seropositive participants returned a negative result in the second serosurvey in Asembo (median age, 23.2 (IQR, 12.5–38.3 years), whereas in Kibera 40/333 (12.0%) sero-reverted (median age, 32.8 (IQR, 23.7–40.6 years). There was no change in serostatus in 161/190 (84.7%) with median age of 21.7 (IQR, 14.2–42.9 years), and 293/333 (88.0%) with median age of 22.3 (IQR, 12.4–36.3 years), of participants who were previously seropositive, and 349/564 (61.9%) with median age, 19.1 (IQR, 9.8–45.4 years), and 175/273 (64.1%) with median age, 12.1 (IQR, 6.7–25.1 years), of those who were previously seronegative, in Asembo and Kibera, respectively. There were statistically significant differences in median ages by change or no change in the serostatus in Asembo. In Kibera, there was age dependent pattern with median age increasing from lowest in those not seroconverting, followed by those seroconverting and those non sero-reverting, to highest in those sero-reverting.
Leveraging on well-characterized populations in two diverse geographical locations, we report heterogenous population exposure based on settings and characteristics at 12- and 16-months following SARS-CoV-2 detection in Kenya. By the third quarter of 2021, about two-thirds of the population in an urban informal settlement in Nairobi, the capital city of Kenya, and close to half of the population in rural Asembo in western Kenya had serologic evidence of SARS-CoV-2 infection. Varying patterns of introduction and transmission of the virus in the two sites could potentially explain the observed differences in the overall seroprevalence—three waves were observed in the sparsely populated rural settings in western Kenya compared to five waves in the densely populated urban informal settlement in Nairobi by 31st December 2021. The timing of virus introduction and transmission varied across the two sites. For instance, the first COVID-19 case was not detected until September 2020 in the rural site and the Delta wave seemed to begin in western Kenya before spreading to the urban informal settlement in Nairobi. The five waves observed in Kibera mirrored the national data22 with all the five variants of concern (Alpha, Beta, Delta, Gamma and Omicron) being detected (unpublished reports, Clayton Onyango). Vaccination coverage in both sites was very low at the time of the serosurveys with only a few (1.2–1.6%) of the study participants having received a COVID-19 vaccine by the start of the third quarter of 2021.
Recent pooled estimates from a meta-analysis of population-based studies show a seroprevalence of 65% in Africa by the third quarter of 202123 and 45% globally24, which is comparable to our estimates in the two populations in Kenya. Considerable increase in seroprevalence was documented after the emergence of new variants, mainly the Beta and Delta variants, in the African and global studies23–25. Following the introduction and spread of the Delta Variant, known to be more transmissible than the earlier variants, we observed a doubling of seroprevalence in Asembo. With vaccination coverage being low during the study period (1.2% in Asembo and 1.6% in Kibera), our seroprevalence estimates were mainly driven by infections. The urban site was yet to fully experience the Delta variant (unlike the rural site) and another serosurvey after the Delta and more recent Omicron variant circulation would likely show considerable increase in seroprevalence across the sites. Other seroprevalence studies in Kenya have documented the remarkable increase in population exposure to SARS-CoV-2 over time9,14,15,26–30. Investigators at Kenya Medical Research Institute in Kilifi reported SARS-CoV-2 antibody seroprevalence among Kenyan blood donors was 49% in March 2021 and 73% in August 202115. Considerable levels of serologic evidence of infection have also been reported for health care workers (43.8% in Nairobi)27, truck drivers (43.3%)28 and pregnant women seeking antenatal care services (49.9% from Kenyatta National Hospital in Nairobi)29 by the end of 2020. Additional seroprevalence surveys in multiple populations after the Omicron wave would be needed to generate a more generalizable estimate of the seroprevalence across the country.
The distribution of SARS-CoV-2 infections is unlikely to be homogeneous across all communities and regions. We observed higher seroprevalence in the urban site than in the rural site for the respective survey rounds. The urban informal settlement environments, such as Kibera in Nairobi, may have been disproportionately affected due to overcrowding, water, sanitation, and hygiene (WASH) infrastructure constraints, and socio-economic challenges that make implementation of COVID-19 public health mitigation measures difficult. Additionally, proximity to the capital city, the main hub for local and international travel, may have led to early introduction and continued population exposure to SARS-CoV-2 especially with the more transmissible variants. A serosurvey in July 2020 in Mumbai, India found the seroprevalence among informal settlement residents to be nearly 3.6 times that of non-informal settlement residents16, which is in line with our findings. The prevalence of anti-SARS-CoV-2 antibodies increased in the urban population from 56% in March 2021 to 64% by June 2021. A previous survey in Kibera in December had reported seroprevalence of 43%9. Most (87%) of the households had been exposed to SARS-CoV-2 by June 2021 in Kibera. Despite the high seroprevalence (albeit with low COVID-19 vaccination coverage) in the urban informal settlement, we observed a high-level of transmission in the fourth (predominated by Delta variant) and fifth (Omicron variant) waves of SARS-CoV-2 circulation, with peak weekly PCR-positivity of 87% in December 2021. In rural western Kenya, the seroprevalence before the second wave was low at 26% but almost doubled after the Delta variant circulation to 49%. The transmission of the SARS-CoV-2 in the third wave was also high in Asembo (peak PCR positivity of 50% in December 2021). The continued transmission in both sites highlights the importance of prioritizing additional mitigation measures, including COVID-19 vaccine distribution in these low socio-economic settings.
An individual’s age and household size were the main predictors for exposure to SARS-CoV-2 infection across the two sites. The lower seroprevalence in children under 10 years of age could be attributable to milder infections in this group, which could be associated with lower antibody titres. Furthermore, adults in both populations would have increased exposure from daily travel to work and/or at workplaces, partly explaining the increased seroprevalences in the adult age groups. Given that the vast majority (87%) of the households had at least one person infected by time of the second serosurvey, the age differences were less obvious, and we expect these differences would dissipate with the recent extensive community circulation of the more transmissible variants such as the Omicron. Close contacts and crowding within the household could explain why the number of household members was an important risk factor for previous exposure. Persons sharing living spaces have increased risk of transmission for respiratory infections within the household, and large households would also have a greater risk of importing infections from outside of the household31.
Though our follow-up of the study participants was limited to an interval of about three months, we observed potential sero-reversions among the previously seropositive individuals. Unlike in Asembo where there were no differences in median ages of those who sero-reverted and the rest, the sero-revertors were older compared to those who maintained their serostatus or seroconverted in Kibera. These observations point to the possible waning of mainly infection-induced immunity, but longer-term follow-up of the cohort would be required to delineate the antibody dynamics and kinetics by age. A longitudinal follow-up of recovered COVID-19 patients showed persistence of neutralizing antibody response beyond six months, though great variations in duration of neutralizing antibody responses (and T-cell responses) that were specific to individual characteristics were observed32. Post-Omicron studies would be required to show the longevity of the antibodies and duration of protective immunity against infection and, disease especially in the context of infection, vaccine-induced or hybrid immunity33,34. Recent studies have shown that the risk of reinfection has been lower in previous Variants of Concern (VOCs) compared with the immune-escaping Omicron variant in previously infected as well as vaccinated individuals35,36. This demonstrates that the presence of antibodies is not a perfect correlate of the level of protection against infection suggesting interpretation of seropositivity status may be challenging. Nonetheless, the presence of SARS-CoV-2 antibodies remains indicative of protection against severe disease and population-based seroprevalence studies would primarily provide reliable estimates of the level of exposure to the infection24,37.
The study is not without some limitations, and some have been highlighted in our early publications9,30. First, selection bias could have occurred as not all individuals from the randomly selected households were enrolled as expressed by age and sex variations in the probabilities for inclusion of the PBIDS population (Figure 3). However, we weighted our population level estimates to account for these variations as described previously9. Second, we did not adjust the reported seroprevalence for assay performance. Diagnostic performance estimates from local or similar populations were missing to provide a meaningful interpretation. Third, some asymptomatic individuals may not seroconvert, some individuals may have been tested prior to seroconversion, and others may have antibodies that had waned by the time of blood collection, so the data in this study may underestimate the true evidence of SARS-CoV-2 infections. Our assay tested for anti-spike IgG antibodies, which show better persistence in serum and may mitigate this problem. Fourth, seropositivity was not confirmed by a neutralization or a secondary assay. Finally, our estimates arise from two underserved populations in Kenya, urban poor and rural communities with limited access to health care and may have limited generalizability in generating national estimates.
In conclusion, we observed continued transmission of SARS-CoV-2 in two diverse populations with high infection exposure and low vaccination uptake in Kenya. The implementation of mitigation measures—such as case identification and isolation, contact tracing and quarantine, and social distancing and uptake of COVID-19 vaccines may have been very challenging in these populations. Despite the high SARS-CoV-2 seroprevalence in urban informal settlements, more transmissible and/or immune escaping variants of concern continued to spread in urban informal settlements. It might be, however, important to prioritize additional mitigation measures, such as COVID-19 vaccine distribution, in these crowded and low socioeconomic settings.
As the dataset contains potentially identifying information on participants, it is stored under restricted access. For more detailed information beyond the metadata and documentation provided, there is a process of managed access requiring the submission of a request for consideration by our Data Governance Committee. Please contact the Data Governance Committee via this email address: GBigogo@kemri.go.ke.
We thank the Kenya Ministry of Health (MOH) and Nairobi Metropolitan Services for granting permission and actively participating in public sensitization for the study. We acknowledge the Kenya Medical Research Institute (KEMRI) provided ethical approval and oversight, field staff that carried out household visit, and the Washington State University Global Health Kenya administration staff that supported the project. This paper is published with the permission of the Director of KEMRI.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Ferrari M, Di Marco L, Pivetti A, Paduano S, et al.: Long-Term SARS-CoV-2 Antibody Seroprevalence in Blood Donors, Italy.Emerg Infect Dis. 2023; 29 (7): 1479-1481 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Hygiene, Infectious dieseas epidemiology, Public Health
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Tropical and Infectious Disease
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious disease epidemiology and serology, with focus on COVID-19.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 09 Oct 23 |
read | read | |
|
Version 1 04 Jul 23 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)