Keywords
Bio-electrical impedance vector analysis, malnutrition, pneumonia, child
Bio-electrical impedance vector analysis, malnutrition, pneumonia, child
In 2017, pneumonia was reported as the leading cause of infectious death for children under five years1. Despite a 30% reduction in global incidence and a 51% reduction of mortality from pneumonia from 2000 to 2015, the implementation of targeted actions to reduce the risk-factors associated with pneumonia mortality is needed to reach Sustainable Development Goals 3.2 by 20302. Paediatric pneumonia fatality rates remain high for children under one year of age, malnourished children and hypoxemic children3,4.
Malnutrition is a key cause of morbidity across the life-course and contributes to a high proportion of under-five deaths5–7. Currently, malnutrition in children is assessed through anthropometric measurements of weight, height and middle-upper arm circumference, with the World Health Organisation (WHO) defining underweight as weight-for-age z-score (WAZ) of <-2, stunting as height-for-age z-score of <-2, and wasting as weight-for-height z-score of <-28. Severe malnutrition, including the presence of bilateral pitting oedema, is considered a danger sign which triggers referral for hospital care amongst sick children9.
However, these traditional anthropometric approaches are not sensitive to cellular health or dehydration, and the presence of oedema will increase a child’s weight and potentially bias malnutrition classification10. As an alternative approach to assessing nutritional status, there is a growing interest in body composition measurements to diagnose and treat malnutrition11,12. Bioelectrical impedance vector analysis (BIVA) is a non-invasive methodology for measuring cellular health13. BIVA is based on passing small electrical currents through the body, giving an indication of the composition of tissues, which can be interpreted to understand the balance between lean mass and fluid.
Specifically one output of BIVA measurements, phase angle (PA), has been found to be correlated with severe acute malnutrition in children12, and with an increased risk of mortality among hospitalized patients14. In longitudinal studies of children with severe malnutrition, BIVA has shown the ability to discriminate children with or without oedema, and to identify loss of excess fluid over time in oedematous children and accretion of lean tissue in non-oedematous children15,16. In one study, children who died had extreme high or low BIVA values at baseline16. However, the extent to which BIVA adds information of clinical value beyond anthropometric measurements remains unclear, and may depend on complications of malnutrition, such as oedematous status.
Given the high morbidity and mortality burden of malnutrition and pneumonia co-presentation, more sensitive approaches to assessing a child’s physiological state could improve outcomes in this group and the use of BIVA in this context is promising. Therefore, we aimed to assess the reliability, clinical utility, and acceptability of BIVA among healthcare workers as an indicator of malnutrition for children under five years of age, with and without pneumonia in Malawi.
We conducted a convergent parallel mixed-methods study, between April and July 2017, in Mchinji District, Malawi. Clinical assessments, anthropometry and BIVA measurements amongst children aged 0–59 months, with and without pneumonia, were collected to explore reliability and clinical utility. Focus group discussions (FGDs) with healthcare workers were conducted to assess acceptability. Synthesis of the mixed-methods was done through Venkatesh and Davis’ Extended Technology Acceptance Model17, which explores a range of attributes of novel technology, including: job relevance, output quality, result demonstrability, perceived usefulness, perceived ease of use, and intention to use.
Ethical approval for the data collection was granted by the University College London Research Ethics Committee (reference: 8075/001) and Malawi National Health Sciences Research Committee (reference: 16/4/1568). The child’s caregiver (including parents, grandparents or older siblings) who was present with them at the hospital provided informed verbal consent for the clinical assessments, anthropometric and BIVA measurements. The verbal consent process was approved by the research ethics committees and was explicitly recorded within the study’s electronic data collection tool; verbal information and consent was used due to literacy levels of caregivers. FGD participants provided informed written consent. The information given to all participants before consenting included the process for data anonymization prior to sharing.
The study was conducted at Mchinji District Hospital, in the central region of Malawi. The hospital provides a secondary level of care, with paediatric and neonatal inpatient wards but limited intensive care capacity. It acts as the main referral facility for the district, which has a population of approximately 600,000, and is mostly rural with a subsistence farming economy. The 2016 Demographic Health Survey reported 10.7% of children under five years were underweight and 32.6% were stunted in Malawi18.
Sampling. Children were recruited using a purposive convenience sampling approach, with the aim to have variation in age, nutritional and HIV status, and equal numbers of those with pneumonia and without pneumonia or another acute infection. We did not conduct a sample size calculation given the exploratory design, but set a pragmatic target of recruiting 50 children with and without pneumonia. Inclusion criteria were being 0–59 months of age and having no previous participation in the study. Children were recruited from the paediatric inpatient ward and outpatient clinic at the hospital (which includes growth monitoring and routine vaccinations). Healthcare workers alerted study staff when children with suspected pneumonia presented and study staff reviewed ward admission notes daily. All children were then screened by study staff for pneumonia using WHO’s Integrated Management of Childhood Illnesses (IMCI) guidelines, enhanced with pulse oximetry9. Healthy children were defined as those who did not have any signs of pneumonia or another acute illness, and were not admitted to the paediatric ward (e.g. children presenting for routine vaccination).
Data collection. Socio-demographic, clinical, anthropometric, and BIVA data were collected by two clinical study staff (BZ and EK), supported by a clinical officer working in the paediatric ward. Staff underwent one-week of training, including: BIVA measurements, refresher anthropometry training, supervised field testing and piloting. All children underwent a clinical pneumonia examination, followed by the anthropometric and BIVA measurements. Other clinical data was extracted from the child’s routine medical records (e.g. malaria and HIV status). Each of the following anthropometric measurements were taken twice, in immediate succession: weight, length (for children <2 years) or height (for children aged 2–5 years) and mid-upper arm circumference (MUAC). Weight was measured using a Salter scale or standing scale, depending on the child’s ability to stand unaided.
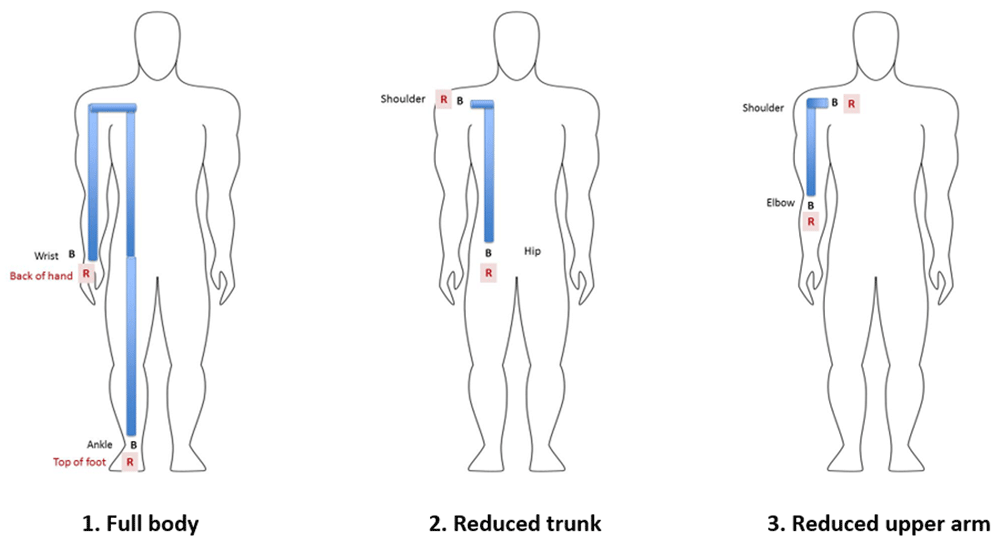
BIVA measurements were taken using the BodyStat 1500MDD battery operated machine. The child was positioned on their back, and sensor pads were placed at the testing locations, with 3cm between electrodes. The following BIVA measurements were taken twice in immediate succession: full body, trunk, and arm (Figure 1). To reduce the movement of children during the measurement, children who were not able to lie calmly were held in place using either a “hugger” (a paediatric immobilizer commonly used for x-rays and scans), or the caregiver held them in position with a folded cotton cloth used as a barrier to the electric current. This pragmatic testing protocol was developed during a piloting period conducted before recruitment for this study, where the influence of movement, position and restraint on measurement results was explored. The following outputs for BIVA were recorded for each measurement site: phase angle at 50kHz, resistance at 50kHz and reactance at 50kHz. The phase angle is calculated as (reactance/resistance)×(180°/π); it represents cell mass and cellular health, with higher values hypothesized to reflect higher lean mass and better cell function, hydration, and higher cell membrane integrity11,14,19.
Analysis. Reliability of BIVA and anthropometric measurements was calculated as the difference between the first and second measurements. All differences between the first and second measurements were converted into positive numbers, and the mean, standard deviation and 95% confidence intervals of the mean for each measure calculated. To investigate the relationship between MUAC and WAZ (exposures), and phase angle for the full body, trunk, and arm segments (outcomes) we conducted linear regression adjusted for age and sex20,21, and stratified by pneumonia status. Children with missing data for any variable were excluded from analyses, as a complete case analysis. Analysis was done using Stata 15.1.
Sampling. The healthcare workers were purposively sampled for the FGDs, with two FGDs planned. The first included community healthcare workers, locally know as Health Surveillance Assistants (HSAs), who had not participated in any other recently on-going pneumonia studies in the district22. The second FGD included healthcare workers working at Mchinji District Hospital, but who had not previously participated in the BIVA study. Participants were invited to take part by phone, following approval to leave their posts from the District Health Officer.
Data collection. The FGDs were facilitated by a local female nurse (EK, ENM qualification) experienced in qualitative research. FGDs were also attended by the project coordinator, a male clinical officer (BZ, Diploma qualification), to introduce the project and answer specific questions relating to the BIVA technology. Both EK and BZ had worked on community and facility-based research in Mchinji District and were present in the hospital for the quantitative data collection, they may therefore have had prior interactions with FGD participants in a research capacity. Prior to the discussions, the participants were given a demonstration on how to use the BIVA machine, an explanation of its function and purpose, and the overall purpose of the study.
The discussion followed a structured guide, with the following topics: current malnutrition assessment methods, burden of malnutrition, initial impressions of the device, improvability, and proposed modifications (see Extended data23). The FGDs were conducted in a mixture of English and Chichewa, based on participant preference and lasted between 25–45 minutes. Discussions were audio recorded following participant consent and then transcribed and translated as a team by BZ and EK (see Underlying data. Field notes were not used, and the transcripts were not returned to participants for checking.
Analysis. The data was analysed thematically using framework analysis by HD24. Framework analysis was selected due to its systematic matrix approach to allow comparison between participants’ viewpoints. After reading through the transcripts multiple times for familiarization, the transcripts were single coded by HD in their entirety using the freely available OpenCode 4.03 software with supervision from CK. The descriptive codes were summarized into categories, and then into inductive themes relevant for addressing the research aim. The transcripts were then re-read to verify that the analytic framework was still reflective of the transcripts. Transcripts were then indexed, and quotes from the FGD participants were charted into the framework. To cross-validate and triangulate the findings, the final interpretation was discussed and agreed between HD, BZ and CK; we did not seek feedback on our interpretations from participants.
Overall, we recruited 52 children (Table 1)25. Although the children with and without pneumonia were comparable by sex distribution, the children with pneumonia were younger than the healthy children (mean age in months: 13.53 vs. 28.57, p-value <0.001). The mean phase angle of the children with pneumonia was lower in all three BIVA measurements compared to the children without pneumonia.
| Total (n=52) | Pneumonia cases (n=24) | Healthy children (n=28) | ||
|---|---|---|---|---|
| Age in months | ||||
| Mean (SD) | 21.63 (17.37) | 13.53 (11.19) | 28.57 (18.84) | |
| <12 months (%) | 20 (39) | 12 (50) | 8 (29) | |
| 12–24 months (%) | 9 (17) | 7 (29) | 2 (7) | |
| 24–59 months (%) | 23 (44) | 5 (21) | 18 (64) | |
| Gender | ||||
| Male (%) | 25 (48) | 12 (50) | 13 (46) | |
| Female (%) | 27 (52) | 12 (50) | 15 (54) | |
| Nutritional Status | ||||
| Height-for-age z-score | -0.84 (1.30) | -0.87 (1.19) | -0.81 (1.39) | |
| Weight-for-age z-score | -0.32 (1.26) | -0.44 (1.43) | -0.22 (1.11) | |
| Body Mass Index (SD) | 16.53 (2.08) | 16.51 (2.61) | 16.54 (1.55) | |
| Underweight (%) | 2 (4) | 2 (8) | 0 (0) | |
| Wasted (%) | 2 (4) | 1 (4) | 1 (4) | |
| Stunted (%) | 8 (15) | 3 (13) | 5 (18) | |
| Disease Status | ||||
| Malaria RDT positive (%) | 5 (10) | 5 (21) | 0 (0) | |
| HIV positive (%) | 0 (0) | 0 (0) | 0 (0) | |
| HIV exposed (%) | 2 (4) | 0 (0) | 2 (7) | |
| HIV unknown (%) | 3 (6) | 3 (13) | 0 (0) | |
| BIVA* | ||||
| Body Phase Angle (SD) | 3.65 (0.62) | 3.43 (0.48) | 3.84 (0.67) | |
| Trunk Phase Angle (SD) | 4.96 (1.07) | 4.69 (0.87) | 5.19 (1.18) | |
| Arm Phase Angle (SD) | 4.19 (0.80) | 3.99 (0.72) | 4.37 (0.83) | |
Table 2 describes the reliability of measurements. Overall, the variation was larger for the children with pneumonia than healthy children for all measures, except weight. For the BIVA measures, the mean difference in the phase angle was similar across the three sites, with a mean difference of 0.20 (95% CI: 0.06, 0.34) for full body, 0.25 (95% CI: 0.17, 0.34) for the trunk and 0.29 (95% CI: 0.18, 0.39) for the arm. Resistance values varied considerably between measurements.
Table 3 shows the adjusted association between WAZ and MUAC with the BIVA phase angle, for each of the three BIVA measurements. The direction of associations between WAZ and MUAC exposures and phase angle were negative for all measures in pneumonia cases, with the strongest associations for trunk measurements (WAZ: aCoeff: -0.261; 95%CI: -0.523, 0.001; MUAC: aCoeff: -0.044; 95%CI: -0.065, -0.024). With the exception of the arm measurement, the direction of association was positive for healthy children, but none were statistically significant.
A total of 11 healthcare workers participated in the two FGDs (five in the hospital discussion and 8 in the community healthcare worker discussion), with all those invited taking part. Through the framework four inductive themes emerged, which are summarized in Box 1: trust in technology, capacity, sustainability, and device usability.
Trust in technology. This theme encompasses the healthcare workers trust and mistrust of the BIVA technology, as well as how they perceived caregivers would accept and trust that appropriate care was being provided.
Based on the demonstration of the BIVA machine, many participants expressed that BIVA would provide an accurate and time saving method to assess the nutritional status of children. There was also trust that with BIVA, malnourished children who are not identified by the current measurements, would be identified given the perception of the device being inherently more accurate.
To the current way of assessing malnutrition sometimes we can measure wrongly and come up with false results and we were telling the child to go home without any help as if the child is ok when in reality the child has a problem, but with this we shall find the real problem (Senior HSA, participant 1)
However, this view was not universal, and some participants expressed hesitation around BIVA due to the lack of knowledge on potential side effects or functionality. They indicated the need for monitoring and evaluation of the technology to be sure it is trustworthy and safe. Similar to their own concerns, the healthcare workers raised the issue of caregivers being worried the device could cause harm, discussing that if a negative perception or myth around BIVA arose it would then be hard to gain their trust. However, other participants said that it would not only be accepted, but it would increase trust in the health system.
BIVA will give them trust that the health workers are really doing science. Unlike the past they could not trust measuring a child using height board (Senior HSA, participant 2)
The communication and terminology used with caregivers was discussed as a key determining factor of whether they would accept BIVA.
Capacity. Capacity emerged in the context of both a barrier and opportunity of BIVA, with the following sub-themes: capacity building through training, increasing capacity through adequate resourcing and self-determination.
Participants in both FGDs voiced the need for education and training on how to use BIVA before they would feel confident. Specifically, the issue of where and how to place the electrodes on the child was addressed by many participants as a something they would need training on. They also voiced the need for community education on BIVA prior to implementation to increase the knowledge of caregivers.
Before we start using it, we need to tell the community, so that when we start using it they will not be surprised, because we informed them, but if we just start without informing them, they will have some fears. (Senior HSA, participant 2)
A reported barrier to current malnutrition assessments was a lack of capacity due to limited resources. This opinion was shared by all participants in the FGD with hospital-based healthcare workers, but wasn’t reflected by the HSAs. Many resources were mentioned as being insufficient – time, tools such as a height board or scale, and knowledge. Some participants stated that BIVA has the potential to solve those problems and increase their capacity to assess malnutrition since currently nutritional status is only being measured by one department in the hospital. However, this was based on the participants’ assumption that BIVA would be made available in multiple departments.
Where I am it happened that it took three days, no seven days for the child to be assessed, just because you have to delegate to another colleague who is also busy, and they said that it was closed and knocked off, if the resources were available in that department that child could have been assessed and treated carefully. (Nurse Midwife Technician, participant 2)
Related to this was a lack of self-determination, with healthcare workers in the hospital FGD expressing concern that due to the overwhelming workload and lack of resources, other healthcare workers don’t make efforts to properly identify and treat malnutrition.
Sustainability. The theme of sustainability includes the sustained practice of implementing BIVA as an indicator of malnutrition, and the physical sustainability of the tool itself. For sustained practice, concerns were raised over how many BIVA machines would be available and who would use them. One participant explained that for BIVA to be successful there would need to be a broad distribution of machines in different wards of the hospital.
The overall concern voiced by almost every participant was that necessary resourcing would need to be secured, otherwise the implementation of BIVA would be unsustainable.
My concern will be the life span of BIVA. How long can we use BIVA because sometimes it can come for the program when it comes to an end you find no more supplies which is also a challenge to go back to old ways of assessing malnutrition. I think it has its expiry date as a machine, unlike the [height] board and MUAC. (Senior HSA, participant 4)
The sustainability of the device was reflected by participants raising questions regarding maintenance and fragility. Most participants also pointed out that the BIVA machine needs constant supplies such as batteries and stickers for the electrodes and running out of either would mean the end of implementation. Despite these barriers, participants overall remained positive that if sufficient resources are available, successful implementation would be possible.
Device usability. The theme of usability includes healthcare workers’ initial reaction to the demonstration, and their ideas to improve the device to make it more user friendly. Almost all participants were positive that the BIVA tool would decrease their workload and be simple to use. For some it wasn’t intuitive, but they felt more confident that it would be a useful tool after seeing the demonstration.
Many participants in both FGDs stated that the BIVA tool would be more user friendly than determining malnutrition using z-scores as obtaining the date of birth and interpreting age-specific results is a key implementation barrier.
Instead of using many tools, for example MUAC, height board, you just use one instead of using more, so I put that BIVA is very easy to use it and we can use it effectively (HSA, participant 3)
Several ideas of how the device could be improved were proposed, the main one being to make the device solar powered instead of using batteries. Participants also expressed a preference for re-usable alternative to the sensor stickers, instead of needing to replace the disposable ones. The facilitators had introduced in their demonstration that the manufacturers were considering alternative ways of attaching electrodes, such as bracelets or cuffs, and many participants were supportive.
One participant proposed that the BIVA machine could be improved with the addition of audio outputs. Another proposed that the measurements would be simplified if children could remain dressed for measurements, which was put forward as time-saving and would make the procedure more acceptable to caregivers of sick children.
The aim of this exploratory study was to describe the reliability, clinical utility, and healthcare workers’ acceptance of BIVA as malnutrition measure for children with and without pneumonia. We observed that children with pneumonia had lower phase angle across all three body segment measurements than healthy children, despite other measures of nutrition being similar between the groups. An interesting finding was the negative relationships with BIVA phase angle for trunk measurements in children with pneumonia, which differed from healthy children when adjusted for age and sex. However, traditional anthropometry had less variation between immediately sequential measurements than BIVA, suggesting usability and practical application may pose challenges.
Healthcare workers expressed trust in this new technology, and thought that BIVA would enable them to diagnose malnutrition more accurately in a time-saving way. This appeared to rest on several assumptions, that the machine’s outputs would be easily interpreted, reliable, and would replace other existing assessments. This was not necessarily triangulated in the quantitative data, with more variability than the traditional anthropometric measurements when two measurements were taken sequentially. We also observed higher variation in the group with pneumonia; it’s likely this was due to the children being younger overall and so less compliant to lying still without restraint, and possibly increased chest movement from effort of breathing. It was also noted by study staff that placing the sensor pads in smaller children and those with cannulas in place could be challenging and would differentially affect reliability in sicker children. Given the burden of severe pneumonia is in younger children3, these operational challenges would need to be addressed.
While we saw this variation between measurements, using an average of two sequential measures is common in some clinical practice. A clear protocol would be needed however, for when a third measure might be needed, or a threshold of difference that would be deemed too unreliable for clinical use. For BIVA outputs to be easily interpreted, a population specific reference needs to be created11. European studies have collected data on large samples of children to make a theoretical model for “healthy” and “unhealthy” phase angle, and the scalability and generalizability of BIVA’s population reference standards varies in literature based on the setting20,21,26. Given the epidemiology of child health is dependent on several context-specific factors (e.g. HIV, malaria, anaemia and nutrition), there would be a need to set not only regional reference standards, but also explore how to differentiate specialized sub-groups, like pneumonia. The fact that BIVA’s outputs would need to be interpreted against population specific references was not presented to FGD participants, and this knowledge may have shaped a more negative perception given the issues raised with interpreting weight in relation to age for current malnutrition assessment.
Results from a study investigating severe acute malnutrition in Ethiopian children supports our finding that sick children have a lower phase angle than healthy children; however, this study reported a significantly positive correlation between MUAC and phase angle in severely malnourished children12. We found the direction of association differed between those with and without pneumonia for full body and trunk phase angle – where a large part of the tissue volume is the lungs. The pulmonary parenchymal inflammation from pneumonia can result in fluid accumulation in the alveoli and swelling of surrounding lung tissue. Depending upon the underlying pathogen(s) airway inflammation and oedema are also typical. We therefore hypothesize that given electrical current faces less resistance in fluid than other denser tissues, a lower phase angle value of the trunk would be expected in children who have pneumonia. Historically chest radiographs have been considered “the gold standard” for the diagnosis of pneumonia in children, with the aim to identify consolidation. However chest radiography lacks sensitivity in differentiating between bacterial and viral pneumonia, are rarely available in low-resource outpatient settings where the majority of pneumonia burden is seen, and require specialized clinical capacity27,28. Therefore, if BIVA can serve a similar function, i.e. indicating fluid in the lungs of sick children, it could provide an alternative differential pneumonia or lower respiratory infection diagnostic.
The main concern raised by healthcare workers was in the sustainability of BIVA, and issues with a renewable power supply, maintenance and continued professional development echo those raised around other technologies in this context22. Several suggestions for improvement and adaption to this context were proposed and provide a good starting point for further development. However, while healthcare workers were unanimous in their desire for improvements in how they assess malnutrition among children, it is still not clear if their endorsement of BIVA was unbiased. The trust that health care professionals had in the new technology after a description and demonstration was high. A study with high-income health care reported that providers can be hesitant to use new technology however29, and the differing perception on welcoming new technology amongst our study participants could stem from the practice of medical device donation. WHO estimate that up to 80% of medical equipment in low-income countries is donated30. Recipients of donations may not feel they have an opportunity or may be hesitant to give feedback to donors on their needs, for fear of losing donations or ruining the relationship with the donor31. The Malawian health care system is heavily reliant on international donations, and therefore this could create a social desirability bias among the respondents who want to maintain the positive relationship with potential donors of BIVA machines and research partners. This likely poses a key limitation in our evaluation of acceptability.
This study had two further key limitations, firstly that we recruited fewer children than initially planned and may have led to under-powered analyses. Our original recruitment plan was aiming for age and sex balance between children with and without pneumonia, however, we struggled to recruit older children admitted to the paediatric ward with pneumonia. Despite adjusting for age in the regression models, this may still account for some of the differences observed. Secondly, we were unable to conduct confirmation chest radiographs and other laboratory measures of cellular health, and therefore it’s possible that some of the children classified with pneumonia did not have lung consolidation.
In this exploratory project, we found a potentially interesting finding around BIVA’s potential clinical utility among children with pneumonia, which warrants further investigation and the creation of population reference standards. The potential for BIVA to provide a quick and non-invasive indication of fluid or differential cellular health in the trunk segment of sick children is worth pursuing, given the challenges in accurate pneumonia diagnosis. However, in its current form, the BIVA device is likely to encounter implementation challenges in a low-resource and high-burden setting.
Figshare: BIVA Malawi Project. https://doi.org/10.6084/m9.figshare.c.605287125.
This project contains the following underlying data:
BIVA_anonymous_analysis.csv (raw data used to conduct the quantitative analysis comparing BIVA and anthropometry measurements).
BIVA_DataDictionary.csv (data dictionary for the dataset used to conduct the quantitative analysis comparing BIVA and anthropometry measurements)
Focus Group Discussion transcripts – BIVA Malawi project.pdf (English translated verbatim transcripts used for qualitative analysis)
Figshare: Bioelectrical impedance vector analysis in Malawi – Supplementary Appendix 1 (FGD topic guide). https://doi.org/10.6084/m9.figshare.1980175623.
This project contains the following extended data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to thank the children and caregivers who took part in the study and gave us their time and information. We would also like to thank the District Health Management Team and clinical staff at Mchinji District Hospital, who supported the conduct of the study - in particular the Clinical Officer in charge of the paediatric ward who contributed to patient recruitment and assessment.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, et al.: Bioelectrical impedance analysis-part II: utilization in clinical practice.Clin Nutr. 2004; 23 (6): 1430-53 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: My area of research is within body composition, with special focus on the BIA-technique in clinical practice.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: malnutrition ; stunting ; child health ; low- and middle income countries ; local produced ready to use foods ; micronutrient deficiencies
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 28 Feb 24 |
read | |
|
Version 2 (revision) 28 Dec 23 |
read | |
|
Version 1 18 Aug 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)