Keywords
systematic review, immunogenicity, pertussis vaccine, whole-cell, children, duration of protection
systematic review, immunogenicity, pertussis vaccine, whole-cell, children, duration of protection
Pertussis (whooping cough), a respiratory infectious disease caused by Bordetella pertussis, and characterized by mild fever, runny nose, and paroxysmal coughing. It is most severe in infants, where the burden of disease is greatest1. Pertussis vaccines have been available globally for decades through national immunization programs2. Two types of pertussis vaccines are available: whole-cell (wP) vaccines composed of killed B. pertussis, and acellular (aP) vaccines based on purified B. pertussis antigens. Pertussis antigens are generally combined in a trivalent formulation with diphtheria and tetanus toxoids (DTP), and often, in addition, with Haemphilus influenza type B (Hib) and hepatitis B virus (HepBV) antigens as tetravalent or pentavalent vaccines. Although aP have replaced wP vaccines in high-income countries, most middle/low-income countries use wP in their childhood vaccine schedules. Globally, vaccine schedules vary significantly. While the majority of low/middle income countries in the Western-Pacific, African and South-East Asian regions use the six-, 10- and 14-week schedule without a booster, Latin American countries use a two-, four- and six-month schedule with boosters at 18 or/and 60 months of age.3,4. More than 100 countries among the 194 monitored by the World Health Organization (WHO) use vaccines that include wP for routine childhood immunization5.
With pertussis outbreaks being reported by many countries in the last decade6–8, additional prevention strategies have been suggested, including maternal immunization, adolescent immunization, and vaccination of close contacts to infants too young to be vaccinated (strategy cocooning). In order to guide decision making, studies modeling the impact and cost-effectiveness of these strategies have been published9. The duration of immunity and protection conferred by childhood wP immunization, one of the parameters to which models are most sensitive, is not known, particularly for wP vaccines currently in use in the world2. In addition, it is still unclear what additional protection is provided by the varying number of booster doses.
Given the importance of understanding the duration of protection from a three-dose primary series, the objective of the present study was to identify, summarize and critically appraise the current evidence on the duration of immunity of currently available wP vaccines in children, for public health purposes and for modelling studies. We also assessed the additional protection conferred by fourth and fifth doses (booster doses), when compared to the three-dose primary series. A review at this time is particularly needed because, in recent years, original manufacturers of wP vaccines have withdrawn from the market and been replaced by new emerging-market manufacturers10,11. Thus, much of the evidence in the literature, which evaluated older products, is no longer relevant.
We first conducted a search to identify currently available wP vaccines and their manufacturers, then conducted a systematic search for published articles that assessed the duration of immunity and effect of boosters of currently available vaccines. The study protocol was registered in PROSPERO (registration number CRD42018107309). We planned and developed the review following the Cochrane Handbook for Systematic Reviews of Interventions12. We followed the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) recommendations for reporting13,14.
The review question, developed in accordance with the elements of the acronym PICO (Population, Intervention, Comparator and Outcomes), is presented in Box 1.
P – Healthy children vaccinated with wP, under 6 years old, of both sexes.
I – Currently available wP vaccine in 3+0, 3+1 or 3+2 dosing schedules.
C –No comparator; children vaccinated with aP; or children vaccinated with different wP dosing schedules.
O – Any outcomes of clinical efficacy or effectiveness, or immunogenicity markers
As there is no single structured source of information on wP vaccines currently in use, we first obtained the 2019 list of wP vaccines pre-qualified by the World Health Organization (WHO)15, which listed five wP vaccine manufacturers. Vaccines from these manufacturers are likely to be used by developing countries as they are commercially available through vaccine procurement funds such as United Nations Children's Fund (UNICEF) and the Pan American Health Organization (PAHO)’s Revolving Fund4. We then obtained the 2018 list of wP vaccine manufacturers from the “Developing Countries Vaccine Manufacturing Network” (DCVMN) from WHO4, which lists 13 manufacturers of wP vaccine, only three of which were also included in WHO’s pre-qualified list. The remaining 10 wP manufacturers likely produce wP vaccines for domestic use by National Immunization Programs, which are thus not commercialized or exported. Finally, we used a January 2018 Global Vaccine Market study commissioned by WHO16, which presents a map of global producers of vaccines that contain diphtheria and tetanus components. Producers that were not on the previous lists (WHO pre-qualified and DCVMN) and could be producers of wP were identified from the map and further verified through internet searches on the websites of each producer. Three more manufacturers were identified from this source.
We included all studies reporting primary data, in which the protection, immunity or duration of immunity conferred by a currently available wP vaccine was assessed. In keeping with the goals of the review, only studies in which immunological markers in a group of individuals were assessed over time after the primary series, or which compared three doses with four or five doses, were included.
Randomized controlled trials (RCTs) and observational studies were included. We categorized observational studies as case-control, cohort (including controlled and uncontrolled cohort studies), and cross-sectional study designs. To be eligible for review, studies had to target healthy children of either sex who had received wP vaccination by the age of six years, and also evaluate a primary schedule of three doses administered by six months of age and/or any additional booster doses. Eligible studies could have compared children vaccinated with wP to unvaccinated children, to children vaccinated with aP, to children vaccinated with different wP dosing schedules, or have no comparator.
The outcomes measured in the study could be clinical efficacy/effectiveness or immunogenicity levels, including seroconversion (% of individuals who seroconverted at a given threshold value), antibody levels (reported as described by the authors as geometric mean antibody concentrations/titers (GMC/GMT)), and/or serological measurements of antibody levels over time. Immunologic markers included anti-pertussis toxin (PT), anti-pertussis fimbriae (anti-PF), anti-pertussis agglutinating antibody (anti-Agg), and anti-filamentous hemagglutinin (anti-FHA).
Studies were excluded if they: assessed children with underlying disease; used fractionated vaccine doses; evaluated only acellular pertussis (aP) vaccine; evaluated only 1 or 2 doses; or had only one immunogenicity assessment after the 3rd primary dose, without any later assessment to evaluate the decay of antibody titers. We also excluded ecological and modelling studies, letters to the editor, recommendations, guidelines, and reviews.
Electronic searches were conducted in Medline (PubMed), Embase, Web of Science, Lilacs, and Central. A complementary search was conducted in the electronic library SciELO. Additionally, reference lists of selected articles and reviews were screened. No date, location, or language limits were placed on the searches which included publications between 30 April, 2019 and updated in September 21, 2021. Search strategies for each database are presented in Supplementary Table 1 (Extended data14).
The procedure for screening and selecting studies was carried out in two phases. In the first phase, three reviewers (AB, MQ and GP) independently screened all titles and abstracts for duplication and inclusion criteria. Then, screened articles were categorized as potentially eligible, unclear, or excluded. Citations on which the reviewers disagreed were discussed or assessed by a fourth reviewer (CT). In the second phase, two reviewers (AB and MQ) independently screened studies obtained for full reading to confirm that they met the eligibility criteria.
Data extraction, done by two independent reviewers, using abstraction forms developed for this review with Microsoft Excel (Microsoft Inc.), included country, funding source, study design, intervention details (vaccine and manufacturer, vaccination schedule, age of administration of each dose), study setting and period, measure of outcome including case definition and diagnostic criteria, baseline information on study population, methods for data analysis, and main results. Results extracted included descriptive study characterization, clinical results (absolute numbers or rates of pertussis cases), and serological results considering any anti-pertussis antibody type, including percentage of individuals who seroconverted and/or antibody GMT/GMC. Finally, information on pertussis vaccines and schedules in use in the country where each study was conducted was also obtained in WHO-reported immunization schedules by vaccine, by country17.
The methodological quality of studies was assessed using: a) the revised Cochrane tool for assessing risk of bias in randomized trials (Cochrane Risk of Bias tool)18, b) the Quality Assessment Tool for cohort or case-control, non-randomized trials studies with: The Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I)19, and c) the Critical Appraisal tools for use in Systematic Reviews Checklist for Analytical Cross Sectional Studies from Joanna Briggs Institute20. For the latter, considering the eight items in the checklist, we categorized the studies as presenting low risk (when scoring 7 or 8), moderate risk (scoring 6 or 5), or high risk (scoring 4 or lower).
Two authors performed the methodological assessments independently, and disagreements between reviewers were assessed and sources of divergence discussed until agreement was reached. When disagreement was not resolved, a third reviewer was used as an arbitrator.
We conducted descriptive analyses of manufacturers and vaccines identified and of published study characteristics including design, country, study period, population, vaccine and schedule used, endpoints considered, number of children enrolled and assessed, and study follow-up period.
For studies reporting immunological outcomes, we originally planned to conduct a meta-analysis to pool results from similar studies presenting antibody titers or seroconversion rates at similar points in time in relation to primary series and each additional booster dose. The meta-analysis was precluded; however, as the included studies were very heterogeneous in terms of the immunological markers and indicators considered, points in time in which they were assessed, and study designs. Thus, we have restricted out findings to a qualitative descriptive synthesis of the results reported by each study.
Results are presented by type of outcome. For efficacy or effectiveness, for studies with clinical endpoints, the main measure of interest is the vaccine’s effect in reducing pertussis, reported as number of events in each study group and absolute risk between vaccinated and unvaccinated as calculated by the review authors. For immunogenicity studies, results are presented either as GMT or GMC of anti-PT, anti-FHA, and/or as percent of individuals who seroconverted given a threshold as reported in the original study. All results are presented by dose of vaccine and timing of measurement of immune response, considering point in time after the primary vaccine series.
In total, we identified 18 manufacturers producing the currently available wP vaccines (Table 1). Seven are located in India, and no other country reports more than one manufacturer; with South Korea being the only high-income country represented. Five of these manufacturers are in WHO’s pre-qualification list – four in India and one in South Korea. Although the WHO list included wP vaccine Quinvaxem, produced by Janssen Vaccines Corp. since 2011, the manufacturer discontinued its production in 2017 and the latest expiry date of batches supplied was December 2019. As this vaccine is currently no longer in use, we did not include it in our analysis.
A total of 2,648 non-duplicate references were identified, and, after screening based on titles and abstracts (first phase), 517 were retained for further assessment. Although the searches were done without language restriction, seven articles had to be excluded because they were written in languages not read by the authors (two Chinese21,22, two German23,24, onw Russian25 and two in Uzbek26,27. After full texts were assessed (second phase), a total of 12 studies28–39 met the inclusion criteria. We present the complete PRISMA flow diagram in Figure 1. The complete list of excluded articles and reasons for exclusion is available upon request.
The included 12 studies report on research conducted in 2007–2020. They evaluated 13 vaccines from only six of the 18 vaccine manufacturers we identified as producing vaccines currently in use. The majority of the included studies (n=8) presented data from vaccines manufactured by Serum Institute of India Ltd (Table 2, Supplementary Table 2, Extended data14). Study designs included three RCT28,29,32, two uncontrolled prospective cohort33,34, and seven cross-sectional studies30,31,35–39. The studies were conducted in seven countries, none of which had introduced maternal pertussis vaccination at the time of study completion (Table 2).
| Characteristic | Number of studies n (%) |
|---|---|
| Manufacturer* | |
| Bio Farma - PT | 1 (8) |
| Microgen Company | 1 (8) |
| Panacea Biotec | 1 (8) |
| Razi Vaccine & Serum Research Institute | 1 (8) |
| Serum Institute of India Ltd | 8 (67) |
| Shantha Biotechnics | 1 (8) |
Study design | |
| Randomized Clinical Trial | 3 (25) |
| Cross-sectional | 7 (58) |
| Uncontrolled Prospective Cohort | 2 (17) |
Publication Year | |
| 2007 | 1 (8) |
| 2008 | 1 (8) |
| 2011 | 1 (8) |
| 2012 | 1 (8) |
| 2013 | 1 (8) |
| 2016 | 2 (17) |
| 2017 | 1 (8) |
| 2018 | 1 (8) |
| 2019 | 2(17) |
| 2020 | 1(8) |
Outcome | |
| Clinical | 2 (17) |
| Immunological | 10 (83) |
Country of study | |
| India | 4 (34) |
| Iran | 3 (25) |
| Palestine | 1 (8) |
| Peru | 1 (8) |
| Russia | 1 (8) |
| Tunisia | 1 (8) |
| Turkey | 1 (8) |
* Sharma H, 201333 assessed 2 different vaccine manufacturers
Detailed characteristics of the included studies are presented in Table 3. Only two studies assessed clinical outcomes35,36, whereas the other studies assessed immunogenicity levels as main study outcomes. For studies that presented individual data over time (all except cross-sectional studies), most had a follow-up time of only one month after a given vaccine dose. Two of the RCT28,29 assessed the first booster dose (equivalent to fourth dose), reporting immunogenicity levels one month later. Three studies34,35,39 assessed children who received a second booster dose (equivalent to fifth dose).
| Study, year | Study design | Country | Study period | Population | Doses (vaccine schedule) | Main outcome assessed | wP vaccine (manufacturer, combination) | Number of children enrolled | Children assessed at end of study | Time of follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sharma HJ, 201129 | Randomized Clinical Trial | India | 2006– 2008 | Healthy infants aged 6–8 weeks at the time of first immunization | 4, NI* | Serological, anti-pertussis toxin (PT) | Serum Institute of India Ltd., DTwP- HepB-Hib | 373 | 304 | 1m after booster |
| Gandhi DJ, 201628 | Randomized Clinical Trial | India | 2012 | Healthy toddlers aged 15–18 months based upon their primary immunization profile | 4, NI | Serological, anti-pertussis toxin (PT) | Shantha Biotechnics Private Limited, DTwP-HepB-Hib | 15 | 15 | 1m after booster |
| Susarla SK, 201932 | Randomized Clinical Trial | India | 2016 | Infants aged 6–8 weeks with a history of being born after normal gestational period (36–42 weeks) with a birth weight >= 2.5 kg and having received birth dose of Hepatitis B vaccine | 3(6-10-14w primary series) | anti- pertussis antibody levels | Serum Institute of India Ltd, Primary series: DTwP-HepB- Hib | 405 | 387 | 4–6 weeks after third dose of vaccination |
| Zarei S, 200734 | Uncontrolled Prospective Cohort | Iran | 2006 | Healthy 4–6 years old children, who had received four doses of DTwP vaccine according to the national vaccination schedule | 5 (2-4-6 primary series and boosters at 18 and 48–72mo) | Serological, anti-pertussis toxin (PT) | Razi Vaccine & Serum Research Institute, Razi-DTwP | 350 | 337 | 1m after booster |
| Sharma H, 201333 | Uncontrolled Prospective Cohort | India | NI | Children aged 15–18 months who had completed their primary immunization schedule | 4 (6-10-14wk primary series and booster at 15–18mo) | Serological, anti-pertussis toxin (PT) | Primary series: Serum Institute of India Ltd, DTwP-HepB-Hib / Booster: Serum Institute of India Ltd, DTwP-Hib | 121 | 118 | 1m after booster |
| Primary series: Panacea Biotec, DTwP-HepB-Hib / Booster: Serum Institute of India Ltd, DTwP-Hib | 108 | 106 | 1m after booster | |||||||
| Cevik, M, 200838 | Cross- sectional | Turkey | 2006 | Healthy 4–24 years individuals who had received four doses of DTwP vaccine according to the national vaccination schedule | 4 (2-4-6 primary series and booster at 18mo) | Serological, anti-pertussis toxin (PT) + filamentous haemagglutinin (FHA) | Serum Institute of India Ltd | 550 | 550 | NA |
| Dashti AS, 201239 | Cross- sectional | Iran | NI | Healthy children aged 2, 4, 6, 12, 18 and 72 months with a valid vaccination record (card), referring to centers for DTwP vaccination | 5 (2-4-6 primary series and boosters at 18–72mo) | Serological, anti-pertussis toxin (PT) | Serum Institute of India Ltd., DTwP | 725 | 284** | NA |
| Bailon, H, 201635 | Cross- sectional | Peru | 2012 | Patients of all ages hospitalized or receiving care due to suspected pertussis, or contacts of suspected cases of pertussis | 5 (2-4-6 primary series and boosters at 18 and 48mo) | Clinical | Serum Institute of India Ltd., DTwP- Hib-HepB / Booster: DTwP | 840 | 191 PCR positive (649 PCR negative) | NA |
| Khramtsov P, 201737 | Cross- sectional | Perm, Russia | NI | Children aged of 2 weeks to 17 years vaccinated according to the National immunization schedule | 4 (3-4.5-6mo primary series and booster at 18mo) | Serological, anti-pertussis toxin (PT) | Microgen Company, DTwP | 135 | 135 | NA |
| Dumaidi K, 201836 | Cross- sectional | Palestine | 2004– 2008 | Infants and children hospitalized with clinically suspected pertussis | 4 (2-4-6mo primary series and booster at 12mo) | Clinical | Primary series: Serum Institute of India Ltd, DTwP-HepB-Hib / Booster: Bio farma, DTwP | 267 | 130 PCR positive (137 PCR negative) | NA |
| Fraj I, 201930 | Cross- sectional | Tunisia | 2018 | Children aged 3–18 years, not having current respiratory infection and visiting the Hospital for check-up. | 4 (2, 3 and 6 mo primary series and booster at 18mo) | Serological, anti-pertussis toxin (PT) | Serum Institute of India Ltd, Primary series: DTwP-HepB- Hib/ Booster: DTwP | 304 | 304 | NA |
| Noel, 202031 | Cross- sectional | Iran | 2016– 2017 | Children at 3 to 15 years, having completed pertussis primary immunization (3 first injections) and with detailed information about history of pertussis immunization in schools | 4 (2, 4 and 6 mo primary series and booster at 18mo) | Serological, anti-pertussis toxin (PT) | Serum Institute of India Ltd, Primary series: DTwP-HepB- Hib/ Booster: DTwP | 1047 | 1047 | NA |
Methodological quality assessment of the included studies and reasons for judgement are presented in Supplementary Tables 3, 4 and 5 in the Extended data14. The quality of the 12 included studies varied significantly.
The two uncontrolled prospective cohort studies had serious33 or critical34 quality issues on Robins-I quality assessment tool (Supplementary Table 3, Extended data14). Of the seven cross-sectional studies, two studies reporting clinical outcomes were assessed as of moderate overall quality35,36. Cross-sectional studies reporting immunogenicity outcomes had heterogeneous quality assessments, with one of high risk37, three moderate risk31,38,39, and two low risk30 based on the Joanna Briggs’ Institute checklist (Supplementary Table 4, Extended data)14. The 3 randomized controlled trials all had an overall high risk of bias (Supplementary Table 5, Extended data)14.
All the RCTs and uncontrolled prospective cohort studies had short follow-up periods28,29,32–34, with a maximum of six weeks post-completion of the primary schedule (third wP dose). Five30,31,37–39 cross-sectional studies included children of older ages (six years of age and older), and only one of these39 evaluated children of 72 months of age who received a second booster dose (Table 3).
We report study results separately for the 10 studies that reported immunogenicity outcomes and the two that reported clinical outcomes (Table 4, panels A and B, respectivelly). For studies reporting on immunogenicity outcomes, GMC levels (EU/mL; 95% confidence interval [CI] or mean ± standard deviation [SD]), and seroconversion percentages (and 95% CI) at each timepoint are described in Table 4 (Panel A). One study33 evaluated two independent cohorts of individuals separately: although both cohorts received the same wP vaccine as the first booster dose, each cohort received a different vaccine in the primary series. We thus report results for these two cohorts separately, resulting in 13 sets of results in total.
| Panel A: Results from studies presenting immunogenicity levels | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, Year | IgG cutoff value considered (EU/ml) | Baseline/ time of evaluation | GMC (EU/mL; 95%CI or mean ± SD) | Number of individuals assessed | Seroconversion N (%; 95%CI) | Follow up/ time of evaluation | GMC (EU/mL; 95%CI or mean ± SD) | Number of individuals assessed | Seroconversion N (%; 95%CI) | Follow up/ time of evaluation | GMC (EU/mL; 95%CI or mean ± SD) | Number of individuals assessed | Seroconversion N (%; 95%CI) |
| Time 1 after primary immunization | Time 2 after primary immunization | Time 3 after primary immunization | |||||||||||
| Randomized Clinical Trial | |||||||||||||
| Sharma HJ, 201129 | 22 | 1 mo post- 3rd dose | 50.87& (45.88 - 56.38) | 152 | 146* (96.06; 91.61-98.54) | 1 mo post- 1st booster | 42.40 (31.40 - 57.25) | 52 | 43* (82.7; 69.67 - 91.77) | NA | NA | NA | NA |
| Gandhi DJ, 201628 | >11 NTU | 1 mo post- 1st booster | 21.8 NTU | 10 | 8 (80; 44.4 - 97.5) | NA | NA | NA | NA | NA | NA | NA | NA |
| Susarla SK, 201932 | any rise in titre post vaccination in comparison with pre vaccination | 4–6 wk post-3rd dose | 9.06 (8.09 – 10.16) | 257 | 238 (92.61,89- 96) | NA | NA | NA | NA | NA | NA | NA | NA |
| Uncontrolled Prospective Cohort | |||||||||||||
| Zarei S, 200734 | 16 | pre-2nd booster (children aged 4–6y) | 8.41& (NI) | 337 | 85* (25.2; NI) | 2–4 wk post- 2nd booster | 30.2& (NI) | 337 | 237* (70.3; NI) | NA | NA | NA | NA |
| Sharma H, 201333 | > 22 | pre-1st booster (children aged 15–18mo) | 9 (NI) | 118 | 64* (53.8; NI) | 1 mo post- 1st booster (children aged 15–18mo) | 42.08 (NI) | 118 | 93* (78.8; NI) | NA | NA | NA | NA |
| 10.3 (NI) | 106 | 52* (49.3; NI) | 1 mo post booster (children aged 15–18mo) | 41.77 (NI) | 106 | 83* (78.3; NI) | NA | NA | NA | NA | |||
| Cross-sectional | |||||||||||||
| Cevik, M, 2008+38 | >24 | post-1st booster (children aged 4–6y) | 15.38&@ (0.5- 48.9) | 87 | 33 (38; NI) | Children aged 7–12y | 67,85&@ (21.4- 127.2) | 162 | 119 (73.4; NI) | Children aged 13–18y | 81.61&@ (40.32-134.08) | 156 | 132 (84; NI) |
| Dashti AS, 201239 | up to mean+2SD | 12 mo of age (post primary series) | 8.58 ±1.08 | 72* | NI | 18 mo of age (pre 1st booster) | 7.35 (1.11) | 85* | NI | 72mo of age (pre 2nd booster) | 14.4 ± 1.06 | 127* | NI |
| Khramtsov P, 2017‡37 | ≥ 100 IU/ml | 6–18 mo of age | 4.7& (1.1–8.4) | 15 | NI | 1–1.5y after 1st booster (children aged 2.5–3y) | 3.2& (0.8–5.6) | 28 | NI | 5–6 y after 1st booster (children aged 6–7y) | 3.5& (1.4 - 8.5) | 27 | NI |
| Fraj I, 201930 | ≥ 40 IU/ml | post-1st booster 3-5 years | 11±3.3 | 55 | 12 (21.8;10.9– 32.7) | NA | NA | NA | NA | NA | NA | NA | NA |
| Noel, 202031 | ≥ 40 IU/ml | post-1st booster 1-2 years after | NI | 42 | 1 (2.4, NI) | NA | NA | NA | NA | NA | NA | NA | NA |
| Panel B: Results from the studies presenting clinical outcomes | |||||||||||||
| Study, year | Case definition | Laboratorial technique | N of PCR positive individuals (after 3 doses) | N of PCR negative individuals (after 3 doses) | N of PCR positive individuals (after 4 doses) | N of PCR negative individuals (after 4 doses) | N of PCR positive individuals (after 5 doses) | N of PCR negative individuals (after 5 doses) | |||||
| Cross-sectional study | Bailon, H, 201635 | Laboratory confirmed cases | Real-time PCR | 6 | 73 | 8 | 83 | 0 | 29 | ||||
| Dumaidi K, 201836 | Laboratory confirmed cases | Real-time PCR | 3 | 5 | 7 | 7 | NA | NA | |||||
EU: Elisa units; GMC: Geometric Mean Concentration; GMT: Geometric Mean Titers; NI: Not informed; NA: Not applicable; Anti-PT: anti-pertussis; AB: Antibody; CI: confidence interval; RP: Prevalence Ratio; mo: months, wk: weeks;
* Estimated by the reviewers
& Results reported as Geometric Mean Titer, IU/mL
+ This study reports serological evaluation in 4 points in time, 3 of which are reported in the table. Results for time 4 after primary immunization (19–24 years of age) = GMT median 77.31 EU/mL (interquartile range 30-263.12).
& Median and interquartile range reported
‡ This study reports serological evaluation in 5 points in time, 3 of which are reported in the table. Results for time 4 after primary immunization (10–11 y after booster) = GMC 33.9 EU/mL (95%CI 10.3–57.5); 23 assessed. Results for time 5 after primary immunization (15-16 y after booster) = GMC 22.3 EU/mL (95%CI 6.6–38.1); 22 assessed.
We analyzed outcomes at five separate time points: up to 30 days post-third dose (primary series); pre-fourth dose/first booster (usually measured at 15-18 months); up to 30 days post-fourth dose; pre-fifth dose/second booster (usually measured at four-six years of age); and post-fifth dose, as depicted in Table 5.
| Study, Year | Post 3rd dose (end of primary series) | Prior to 4th dose/1st booster | Post 4th dose/1st booster | 4-6 years of age (before 5th dose/2nd booster) | Post 5th dose% (after 2nd booster) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| GMT | Seroconversion | GMT | Seroconversion | GMT | Seroconversion | GMT | Seroconversion | GMT | Seroconversion | |
| Sharma, 2013aα 33 | X& | X | X& | X | ||||||
| Sharma, 2013bα 33 | X& | X | X& | X | ||||||
| Gandhi, 201628 | X& | X | ||||||||
| Zarei, 200734 | X& | X | X& | X | ||||||
| Sharma, 201129 | X | X | X | X | ||||||
| Cevik, 200838 | X | X | ||||||||
| Khramtsov, 201737 | X (at 6- 18m) | NI | X (at 2.5-3 yrs) | NI | X (at 6-7 yrs) | |||||
| Dashti, 201239 | X (at 12m) | NI | X | X (at 6 yrs) | ||||||
| Fraj I, 201930 | X | X (at 3-5 yrs of age) | ||||||||
| Susarla SK, 201932 | X | X | ||||||||
| Noel, 202031 | X (1-2yrs after) | |||||||||
α Sharma H. et al. (2013)33 assessed 2 cohorts of children. We thus report on each cohort individually.
& GMT measures available but no SD or 95%CI.
NI: Not informed
The data show that immunogenicity levels, reported as mean GMT, were low right before the fourth (first booster) with all studies reporting levels below 11 EU/mL33,37,39, with three reporting values reaching above 40 EU/mL one month after the fourth-dose studies29,33 (Figure 2). Similarly, low levels of GMT were reported prior to the fifth dose34,37–39, with the single study evaluating pre and post-fifth dose reporting levels of 8.4 EU/mL prior, and 30.2 EU/mL 30 days post booster34.
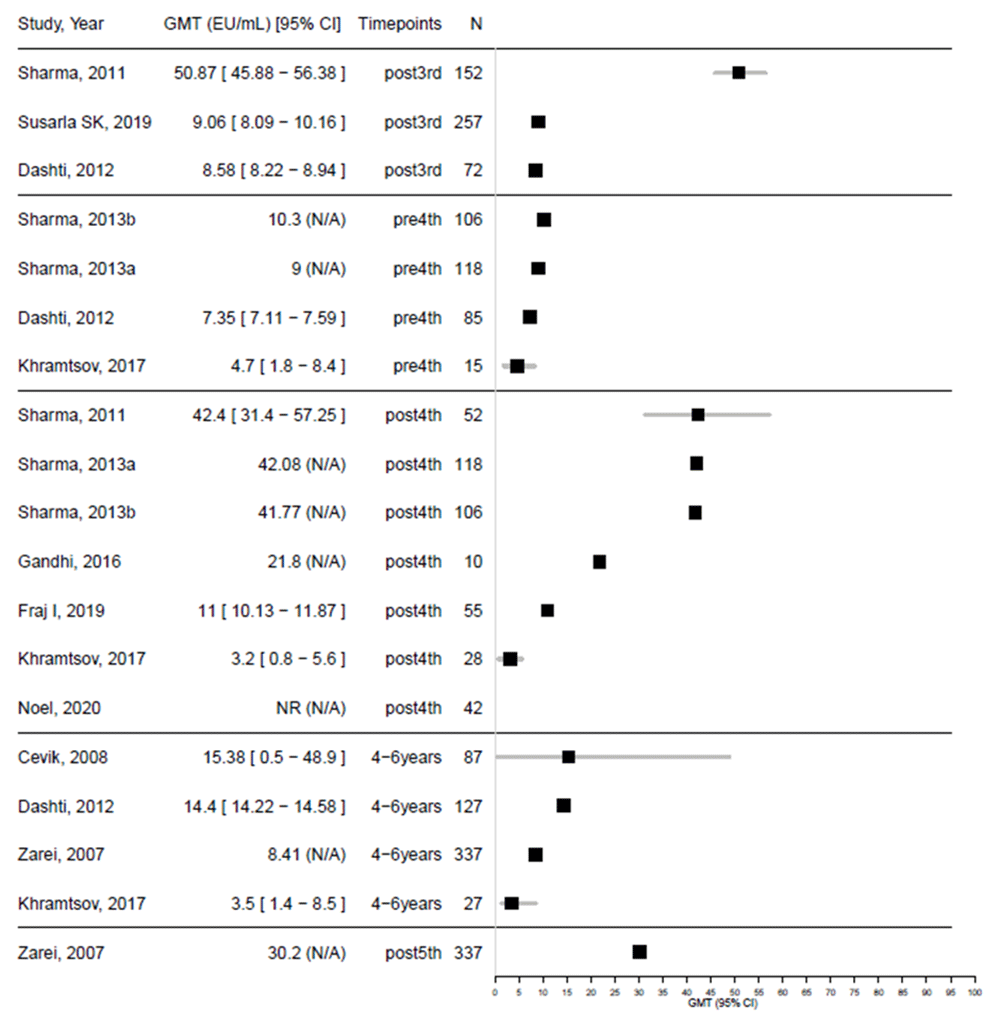
When looking at seroconversion rates (Figure 3), higher (over 75%) seroconversion was observed after completion of the three-dose primary regimen, after the first booster28,29,33, and after the second booster34. The only two studies that reported low levels of serocoversion rates post-fourth dose30,31 are those that evaluated serocoversion post second booster later in time (at least one year after the booster), at three-five years of age30, and one to two years after the booster31.

The results viewed in combination suggest that immunogenicity may wane substantially after the primary series and between booster doses.
How immunogenicity correlates with clinical outcomes is not totally clear. In the two cross-sectional studies reporting clinical outcomes (Table 4, Panel B), infection rate of PCR-confirmed pertussis in children was heterogeneous, with 7.6% (6/79) post-third dose and 8.8% (8/91) post-fourth reported by Bailon et al.35, and 37.5% (3/8) post-3rd dose and 50% (7/14) post-fourth reported by Dumaidi et al.36.
We conducted a systematic review of the published literature on the effectiveness of whole-cell pertussis (wP) vaccines currently used in childhood vaccination programs to address two related issues: the duration of immunity conferred by currently available wP vaccines and the efficacy of booster doses. These issues are especially important as much of the evidence in the literature evaluated older and no longer available vaccines. Many traditional manufacturers have withdrawn from the market and new vaccines from emerging-market manufacturers of wP vaccines have entered in their place11,40.
We identified 18 manufacturers that provide wP vaccines currently in use and 12 studies that evaluated the efficacy of some of those vaccines. The first important result to highlight is that there is thus no published information about many of the vaccines currently in use worldwide.
Current pre-qualification and registration requirements request only that pre-licensure studies of combination vaccines demonstrate non-inferiority to existing vaccines over a short follow-up period (usually 30 days). Longer-term studies of the duration of immunity are not required by licensing authorities and are no longer routinely conducted3. For the vaccine products for which we identified published studies included in this review, many did not directly address duration of immunity, due to the current pre-qualification and registration requirements described above3. We attempted to glean some information about duration of immunity by examining the pattern of outcomes just before and after booster doses. Serological measures indicated low immunity just before administration of booster doses, and high immunity in the 30 days after they were administered, suggesting that the duration of immunity after the three-dose primary series may not be long, perhaps only a few years. This conclusion is, however, only suggestive because of the short follow-up periods in these studies, and the fact that the majority of them presented with high or moderate risk of bias.
It has been thought for some time that anti-pertussis antibody levels rapidly wane over time41 and the pattern in these 12 studies supports this hypothesis, as demonstrated by high GMC and seroconversion rates observed shortly after vaccination, but lowering levels over time. Three published meta-analyses reported on the clinical efficacy and effectiveness of wP and aP vaccines, reaching similar conclusions42–44. Fulton et al. conducted a meta-analysis to evaluate the clinical efficacy or effectiveness over three years of childhood vaccination of a primary series of then-available vaccine formulations (wP and aP), reporting an overall effectiveness of 94% for both aP and wP vaccines. The protective effect was lower for aP when compared to wP vaccines42. McGirr et al. evaluated and compared the duration of protective immunity from childhood immunization series with three or five doses of then-available diphtheria-tetanus-acellular pertussis (DTaP) vaccines44. McGirr estimated that protection from aP vaccines dropped to only 10% after 8.5 years of the last dose received. A recently systematic review of Wilkinson et al.45 evaluated the effectiveness and duration of protection from both aP and wP, concluding that pertussis vaccines confer protection in the short term against disease, with protection waning rapidly for aP vaccine. This review included 22 studies reporting on wP vaccine and reported an overall effectiveness against disease of 79% (I²= 93%) in metanalysis. However, this study included many vaccines no longer available, and as opposed to our study, did not limit the analyses to vaccines currently in use.
Duration of immunity is key to establishing optimal vaccination strategies and one of the parameters to which dynamic models of pertussis are most sensitive. Models have used values varying from 10 to 75 years to represent the duration of immunity from wP46. This wide range of assumptions clearly show a lack of consensus with regards to this estimate, likely resulting from a lack of good evidence in the literature. Though models can explore a wide range of assumptions and thus suggest how this uncertainty may impact the study findings, it nevertheless highlights how the projection of the consequences of vaccination strategies is impaired by lack of robust evidence on the duration of immunity.
Accurate estimates are more challenging to identify for pertussis vaccines as there is no established immunological correlation between protection against pertussis47 and immunogenicity data, which is the outcome reported in 10 of the 12 studies included in our analysis. Different wP vaccines may have different antigenic content, leading to variations in post-vaccination immune response. However, as pointed out by the WHO’s Strategic Advisory Group of Experts (WHO SAGE) working group on pertussis vaccine, patterns of immune response may contribute insights on vaccine effectiveness48, and despite the lack of serological correlates for protection, the United Kingdom Medical Research Council suggested a correlation between high agglutinin titers and clinical protection49. Furthermore, epidemiological observations also suggest that the protection of pertussis vaccine is high only for a limited period and falls gradually with time after immunization3. Our study of currently available wP vaccines corroborates those observations.
Our study has several limitations. First, identifying all currently available vaccines was difficult because of the market changes over time and the merging and incorporation of vaccine manufacturers globally in this period49. Second, due to current licensure and pre-qualification requirements, most studies were not randomized clinical trials nor prospective cohorts of immunized children, but rather cross-sectional or immunogenicity studies, the former with no follow-up and the latter with very short follow-up. In cross-sectional studies, groups of children at various ages are assessed at one timepoint; interpretation of antibody titers and their relation to vaccine doses is difficult since natural infection also influences antibody titers.
Taken together, our results and the limitations highlighted above suggest that the way current wP vaccines are assessed hinders the adequate evaluation of the duration of immunity and protection over time. The scant evidence available from this and other studies suggests that the immunity conferred by currently available wP vaccines may be relatively short, perhaps only a few years, but that suggestion is based on studies of short duration and poor design that used an unreliable measure of immunity. Considering that wP vaccine formulations are widely used globally, to protect millions of children each year, careful consideration and planning needs to be given to alternative approaches for monitoring duration of immunity and for developing reliable correlates of protection. Thus for researchers, alternative strategies for evaluating the duration of wP protection and immunity are needed; they may involve developing new ways of using epidemiological observation studies and surveillance systems to track pertussis outbreaks, guide vaccination efforts, and promote the development of vaccines with more durable effects.
All data underlying the results are available as part of the article and no additional source data are required.
Harvard Dataverse: Systematic review of immunogenicity and duration of immunity of currently licensed wP vaccines in children – Supplementary Files. https://doi.org/10.7910/DVN/XFRXGA14
This project contains the following extended data files:
- Figure 1- PRISMA 2020 flow diagram.
- Figure 2.png
- Figure 3.png
- Supplementary Table 1. Databases searched, electronic search strategy, and dates last searched by database.docx
- Supplementary Table 2. Whole-Cell Pertussis vaccines (manufacturer, commercial name and country of origin) included in the Systematic Review.docx
- Supplementary Table 3.docx
- Supplementary Table 4.docx
- Supplementary Table 5.docx
Harvard Dataverse: PRISMA flowchart and checklist for ‘Systematic review of immunogenicity and duration of immunity of currently licensed wP vaccines in children’. https://doi.org/10.7910/DVN/XFRXGA14
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The authors are grateful to Gabriel Cardozo Muller, who prepared the forest plot figures.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
References
1. Rohani P, Drake JM: The decline and resurgence of pertussis in the US.Epidemics. 2011; 3 (3-4): 183-8 PubMed Abstract | Publisher Full TextCompeting Interests: I received consulting fees from GSK for discussions about pertussis vaccines. I confirm that this potential conflict of interest did not affect my ability to write an objective and unbiased review of the article.
Reviewer Expertise: Infectious disease epidemiology
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vaccinology, public health, epidemiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 05 Aug 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)