Keywords
pregnancy, placenta, rapid review, pathology, ultrasound
pregnancy, placenta, rapid review, pathology, ultrasound
It has long been known that the placenta is a complex organ with a vital role in all aspects of fetal growth and survival including gas exchange, nutrient transport, hormone synthesis and protection against pathogens1,2. Despite its immense importance, researchers have recognized it as the “least understood human organ”3 and “multitalented, but still mysterious”4. These acknowledgements spurred the establishment of the Human Placenta Project by the National Institutes of Health in 2014 to increase research particularly relevant to in utero placental development5.
A large body of research links complications of pregnancy, including preeclampsia, intrauterine growth restriction, and preterm birth to disorders of placentation, affirming the significance of placental health to the health of the pregnancy and fetus6–8. Furthermore, women with placental disease such as maternal vascular malperfusion are at higher risk of developing cardiovascular disease later in life9,10. The importance of the placenta in the health of the offspring is evidenced in connections between placental morphology (such as placental surface size, placental weight, and maternal cotyledons) and childhood hypertension11, asthma12, and disorders of eye development13, as well as cardiovascular disease and obesity later in life1. The placenta’s role in chronic disease outcomes has become central in the developmental origins of health and disease hypothesis14.
While there is extensive research showing the associations between placental characteristics and a wide range of pregnancy disorders and maternal and child health outcomes, to our knowledge, the extent of all pregnancy research that incorporates data on the placenta has not been evaluated. The primary aim of this rapid review was to quantify the percentage of human pregnancy studies that include placenta data. We secondarily aimed to categorize and describe the placenta data being reported.
We conducted a rapid systematic review of published pregnancy studies, closely following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines15 but limiting the scope to be more rapid in nature: we searched one database (PubMed) for articles of research in the US published within a one-year period (2018). The search strategy for PubMed was the following:
"pregnancy"[MeSH] AND (Observational Study[ptyp] OR Controlled Clinical Trial[ptyp] OR Randomized Controlled Trial[ptyp] OR Multicenter Study[ptyp] OR Comparative Study[ptyp] NOT Review[ptyp] NOT Meta-Analysis[ptyp] NOT Systematic Review[ptyp] NOT Case Reports[ptyp] NOT Letter[ptyp] NOT Comment[ptyp]) AND ("2018/01/01"[PPDAT] : "2018/12/31"[PPDAT]) AND "humans"[MeSH Terms] AND English[lang] NOT Africa[MeSH] NOT Asia[MeSH] NOT Central America[MeSH] NOT South America[MeSH] NOT Latin America[MeSH] NOT Caribbean Region[MeSH] NOT Europe[MeSH] NOT Islands[MeSH] NOT Oceania[MeSH] NOT Canada[MeSH]
The last search was completed on November 27, 2019. We did not register this rapid review in PROSPERO due to time constraints, and this review did not require ethical approval.
Peer-reviewed, original human research focusing on pregnancy in the second trimester, third trimester, and/or labor and delivery were included in this search. Studies were eligible if published between January 1, 2018 and December 31, 2018, according to the publication date available in PubMed. Studies had to be conducted in the United States and published in English. If the study location was not specified in the publication, author affiliations were checked for locations and deemed eligible if all or most authors had affiliations in the United States.
Case reports and review articles were excluded, as well as in vitro studies or animal models. Studies confined to periconception or the first trimester of pregnancy (up to 12 weeks gestation) were also excluded. Because there is limited ability to study the developing placenta in vivo in humans during this early gestational time, we did not want to overinflate the percentage of pregnancy studies that did not include the placenta.
We used Rayyan (Qatar Computing Research Institute, Doha, Qatar) to manage articles and record decisions during the review process. Three independent researchers (LAT, KG, KAO) reviewed articles at both the abstract and full article review stages, applying inclusion and exclusion criteria at both stages. If disagreements arose, all reviewers discussed the issue and formed a resolution, consulting with a fourth investigator (ADG) as needed. We did not appraise the quality or assess risk of bias of individual studies because this was beyond the scope of this rapid review.
The same three reviewers extracted data from the publications and compared results. If disagreements arose, all reviewers discussed the issue and formed a resolution. The following information was extracted from the publications: first author’s last name, journal title, study design (randomized controlled trial, cohort, case control, cross-sectional), inclusion of placenta data (yes/no), time of placenta data collection (during pregnancy or after delivery), method of data collection (i.e., ultrasound, MRI, pathology), type of placenta data reported (i.e., placenta weight, estimated placental volume, placental abruption, etc.), and study objective(s). To determine if placenta data was reported, the authors searched each article in its entirety for “placenta” and related terms. We looked for any indication that placenta data was collected and reported. Study objectives were categorized into four groups: outcomes related to mother, infant, placenta, or a combination of 2–3 outcomes. Extracted data was recorded in an Excel (Version 2008) spreadsheet, in which all counts and percentages were calculated.
A total of 1,448 publications were identified in the PubMed search. After screening titles and abstracts, 363 publications underwent full article review, and 290 studies met all eligibility criteria (Figure 1). A total of 42 studies reported placenta data in some capacity.
Over half of the total studies included in this review were cohort designs, almost a third were randomized controlled trials, and less than 10% were case-control studies or cross-sectional studies (Table 1). The proportion of studies within each study design was similar for those with and without placenta data compared to total studies, but a higher percentage of studies with placenta data tended to be observational designs compared to those without placenta data. Sample sizes covered an extremely wide range from very small to tens of millions. The range was 8 to 57 million subjects for all studies, 10 to 57 million for studies with placenta data, and 8 to 42 million for studies without placenta data.
Across all studies, 47% had study objectives targeting one or more outcomes solely related to the mother; over a third had outcomes related solely to the infant, and 17% examine outcomes for both mother and infant (Figure 2). Only one study had a placenta outcome. Among the subset of 42 pregnancy studies that reported placenta data, over half were studies with infant outcomes. Finally, in the group of studies that did not report placenta data, the percentage of outcomes in each category was similar to the findings for all studies, with approximately half focused on maternal outcomes.
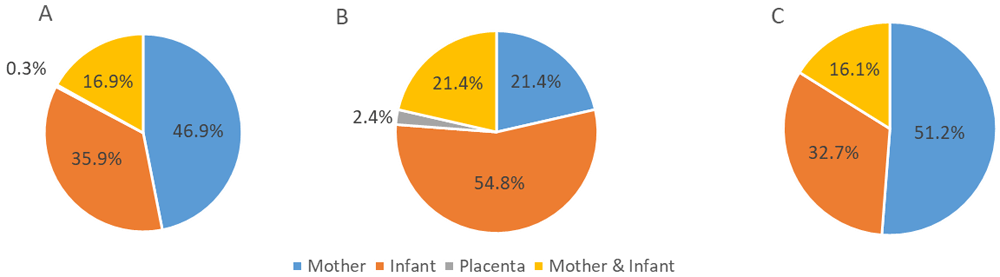
Categorization of study outcome(s) of human pregnancy studies conducted in the United States and published in 2018: (A) all studies (n=290); (B) studies that report placenta data (n=42); and (C) studies that do not report placenta data (n=248).
Of the studies with placenta data, 29 reported data collected during pregnancy, and 19 studies reported data collected after delivery (Figure 3). Five of these studies included placenta data from both pregnancy and postpartum. During pregnancy, 16 studies collected placenta data via ultrasound, 1 study collected placenta data via MRI, and 13 studies used other proxy methods for direct placenta measurements, such as clinical examinations and chart reviews (Table 2). After delivery, 13 studies collected placenta data via pathology examinations, and 8 studies collected placenta data via other methods (e.g., umbilical cord blood collected). Some data collection methods were unspecified (n=6).
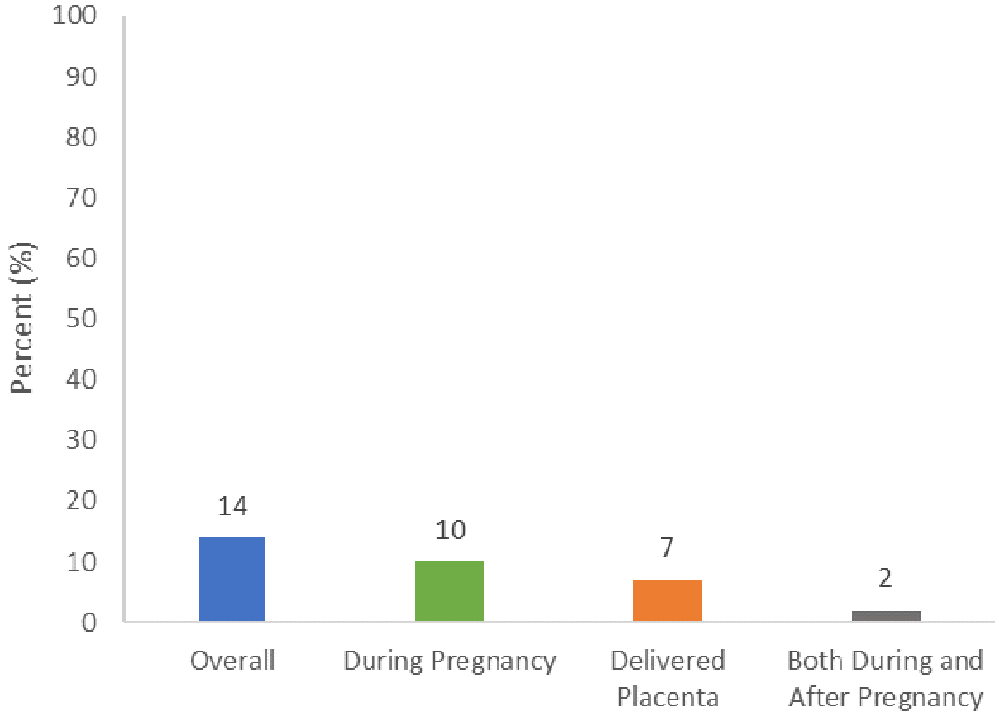
Studies that report placenta data collection at both time points are counted more than once.
| Time and Method of Placenta Data Collection | N (%)1 |
|---|---|
| During Pregnancy | 29 (69) |
| Ultrasound | 16 (38) |
| MRI | 1 (2) |
| Other | 13 (31) |
| Delivered Placenta | 19 (45) |
| Pathology | 13 (31) |
| Other | 8 (19) |
We conducted a systematic review to quantify the percentage of human pregnancy studies that include placenta data – limiting the scope to those conducted in the United States and published in 2018 to facilitate a rapid review. Fourteen percent of the studies reported placenta data, most of which was collected by ultrasound during pregnancy or through pathology exams after delivery. More studies that reported placenta data were focused on infant outcomes compared to studies without placenta data, which were focused more heavily on maternal outcomes. Only a single study, out of 290, focused on outcomes related directly to the placenta.
The placenta’s invaluable role in pregnancy is undisputed - it dictates the success of growth and development in a pregnancy through a range of processes including nutrient sensing and endocrine signaling between mother and fetus. Abnormal placental development and dysfunction, along with fetal insults in utero, have been shown to impact the growth and development of offspring across the lifespan14,16. Assessing the placenta both during pregnancy and after delivery can shed light on the pathophysiology of adverse outcomes, including clues to early or late gestation insults. Scifres et al. found that in pregnant women with gestational diabetes mellitus, maternal vascular malperfusion lesions in the placenta were associated with excess gestational weight gain and lower infant birth weight, as well as increased risk of preterm birth and hypertensive disorders of pregnancy17. Similarly, Hauspurg et al. found that the presence of placental maternal vascular malperfusion in healthy pregnancies was associated with increased risk of adverse outcomes in later pregnancies18. In a cohort study of over 900 pregnant women, Salafia et al. found that placental disk size, including chorionic surface shape area and perimeter, was correlated with infant birth weight and gestational age at delivery19.
However, assessing the placenta often requires extensive training, is time-consuming, and can quickly become expensive. Dimitrova et al. found that additional specialized training was needed to detect ultrasound indicators associated with placenta accreta spectrum disorders, compared to basic obstetric ultrasound training20. Some researchers have begun working to make placenta data collection easier, more common, and automated. Salafia and colleagues have used placenta images to examine variations in surface shape and vascular development, and how these can indicate the presence of maternal and fetal vascular pathologies21. Our group is working to develop rapid placenta assessment software based on photographs using artificial intelligence methods22. This and other work could help current and future pregnancy research to more easily include the placenta in pregnancy studies.
In our aim to assess the proportion of pregnancy research that utilizes the placenta, we chose to conduct a rapid systematic review as a first step toward this goal. Rapid reviews have become a helpful way to gather broad information and assess various topics, including those in healthcare. A rapid review can be conducted quickly, does not require multiple independent reviewers (although we did use multiple reviewers), and can provide broad descriptions and information of detailed topics23. Hummel et al. conducted a qualitative rapid review to evaluate ethical problems in healthcare for pregnant women in epidemics24. This review was able to quickly identify common healthcare-related risks and issues through a targeted database search, and qualitatively assess the proposed management plans for each. Antony et al. conducted a rapid review commissioned by the World Health Organization to evaluate the efficacy of quality improvement plans on patient safety in obstetrics25. Their review found that combined healthcare provider education and quality improvement plans could improve maternal and newborn safety during delivery. Most recently, researchers have conducted rapid reviews of Coronavirus Disease 2019 (COVID-19) during pregnancy to provide quick results during a rapidly spreading pandemic26. Our rapid review, similar to others, allowed us to examine a broad topic and a large number of studies without the constraints of traditional systematic reviews.
A major strength of our study was the rigorous systematic review process, guided by a PhD-level university librarian with expertise in health sciences literature. Additionally, this review was conducted by three reviewers, ensuring cross-checking of eligibility criteria and consistency in reviews. The main limitation of our work was the shorter time to complete the review. Due to this self-imposed constraint, we narrowed the scope of our review to include only one year of publications within a single database, which undoubtedly reduced the number of studies available for review. A drawback was that not all pregnancy studies provided detailed methods or gestational timing for the placenta data collection, limiting our ability to describe the studies in our assessment.
The placenta is not only of immense importance in each and every pregnancy, it is often the key to understanding short- and long-term outcomes for both mother and child. In this rapid review, we found that only a small proportion of pregnancy studies report placenta data in research, and it is rare for human pregnancy studies in the US to focus on outcomes related directly to the placenta. Future systematic reviews could expand the publications years and locations of research or alternatively focus on methods papers to ascertain whether placenta data is being collected but not reported in analysis. Overall, this paper quantifies the low percentage of pregnancy studies that include the placenta and adds to the many publications highlighting the dearth of placenta research. Pregnancy researchers across all disciplines should aim to include the placenta in studies of maternal and infant outcomes.
ScholarSphere (Penn State): Placenta Rapid Review Data Extraction_Scholarsphere for ‘How often is the placenta included in human pregnancy research? A rapid systematic review of the literature’, https://doi.org/10.26207/857e-0b7327.
ScholarSphere (Penn State): PRISMA checklist for ‘How often is the placenta included in human pregnancy research? A rapid systematic review of the literature’, https://doi.org/10.26207/0d78-9p5328.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We would like to acknowledge Dr. Christina Wissinger for her expert guidance in choosing the rapid review method and finalizing the search strategy. We would also like to acknowledge Celeste Beck for her overall support and guidance through the review process, as well as her assistance with using Rayyan for study selection.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: We are conducting a similar analysis but on a broader aspect of reproductive biology.
Reviewer Expertise: Bioinformatics, computational biology, pregnancy, placental biology and development, preeclampsia, genetics
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Developmental origin of diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 03 Mar 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)