Keywords
Neutrophils, Tuberculosis, NETosis
Neutrophils, Tuberculosis, NETosis
No major differences were made from the original version of the manuscript, except for the clarification on study participants and samples in the method sections.
See the authors' detailed response to the review by Delia Goletti
See the authors' detailed response to the review by Ashwani Kumar
Tuberculosis is a disease caused by Mycobacterium tuberculosis (Mtb) and is responsible for approximately 1.5 million deaths annually1. Understanding lung immunity during TB progression is important in identifying biomarkers. During initial TB infection, an immune structure known as a granuloma forms to control and prevent the spread of Mycobacterium tuberculosis2. The granuloma is a highly organized immune structure composed of macrophages surrounded by a layer of epithelioid cells and multinucleated giant cells, with lymphocytic cuff3. Neutrophils are also found in the regions adjacent to caseum or necrotic regions of the granuloma4. Understanding the contribution of neutrophils to lung associated tissue damage is important in determining mechanisms that lead to TB associated pathology. Neutrophils are the most abundant cell subsets in the lung and are also amongst the first cells that are infected with Mtb4. Neutrophils play an important role in bacterial control during acute infection, however, they can release reactive oxygen species (ROS), deoxyribonucleic acids (DNA), myeloperoxidase, cathelicidins and S100 proteins that can result in excessive inflammation and damage to surrounding tissue5,6. Netosis is the release of nuclear DNA and associated proteins i.e. histones, called neutrophil extracellular traps (NETS) from the cell in response to bacterial, viral or stress induced conditions7. The size of the NETS released is dependent on the size of the pathogen8, which is important in combatting infection. However, netosis has also been shown to contribute to tissue damage in various diseases9–11. Studying neutrophils in a clinically relevant environment is challenging due to the short life-span and the various complex triggers of activation, including respiratory stress, damage-signalling or prior activation to pathogenic stimuli resulting in ROS associated activities12.
Neutrophilia, which is a characterized by an abundance of neutrophils above what is considered to be a normal count, has been associated with TB and it is therefore important to understand mechanisms underlying neutrophil mediated contribution to disease pathogenesis13,14. In previous findings, we reported that across cellular, caseous and cavitary granulomas, proteins associated with neutrophils were abundant in necrotic regions15 and in the border of necrotizing tissue5. However, whether neutrophils are directly causing caseation and how it is mediated remains a gap in our knowledge. Our project is therefore aimed at determining expression profiles of neutrophil associated genes in blood from healthy, LTBI and TB participants as potential markers of the disease. We also intend to investigate the role of neutrophils during TB pathogenesis by exploring, in-vitro, the dynamics of NETosis, in neutrophils infected with Mtb and to target drivers of the cell death as host directed therapies (HDT).
This is a prospective study that will include participants recruited from healthcare facilities that service the eThekwini District in KwaZulu-Natal (KZN), South Africa. Participants will be recruited from Kwadabeka Clinic and Prince Zulu Communicable Disease Centre in Durban. South Africa has a TB prevalence rate of 737 in 100 000 in 201716 with eThekwini described as having the highest TB prevalence rate in KZN. This study was approved by the Biomedical Research Ethics Committee (BREC) at the University of KwaZulu-Natal (BE022/13 and BE00003365/21).
A qualified nurse will enrol participants into the study after explaining the informed consent procedure according to good clinical health practices (Figure 1). Individuals who are newly diagnosed with active TB, determined as GeneXpert positive and who have not received treatment, will be recruited to the TB group of the study (n=30). Individuals who are healthy will be recruited to the healthy group as determined by their quantiferon (QFT) results. If the QFT result is positive those individuals will be recruited to the LTBI group (n=30) and those who are QFT negative will be recruited to the healthy group (n=30). Participants will be incentivised for participating in the study according to the ethics that have been approved through the University of KwaZulu-Natal. Participants will be informed that their biological samples will be stored for future use, including their genetic information, which will be used for research. A volume of twenty millilitres of blood will be used for neutrophil isolation. Plasma will be collected and stored at -80°C (Figure 2).
Samples obtained will be assigned a unique participant identification. A maximum of 40 ml of blood will be drawn from a participant. 10 ml of blood will be stored for paxgene processing in order to isolate RNA for genetic studies. 20 mls of blood will be collected and used to isolate peripheral blood mononuclear cells (PBMCs) and the remaining 10ml of blood will be used to collect plasma.
A volume of blood ranging between ten and twenty millilitres of blood will be collected in heparin collection tubes (Figure 2). The blood will be transferred to 50 ml conical tubes. A volume of ten millilitres of 0.01 M phosphate buffered solution (PBS) will be added to the blood collection tubes, to remove any blood remnants and then added to the 50 ml conical tube. A volume of twelve millilitres of the Cell-based Assay Neutrophil Isolation Histopaque (Sigma-Aldrich, Germany) will be placed in a new conical tube at room temperature (RT). The diluted blood will be pipetted on top of the histopaque and centrifuged at 800 x g for 20 minutes at RT, with the centrifuge setting of acceleration slow and deceleration off. The yellow and clear upper layers will be aspirated, leaving only the red blood cell (RBC) layer and neutrophils. A volume of twenty-five millilitres of the RBC lysis buffer (1x solution) will be added to the RBC and neutrophil layer and mixed. The mixture will be incubated at RT for 10 minutes. Following incubation, the cells will be centrifuged at 250 x g for 5 minutes at RT to pellet the neutrophils. The supernatant will be aspirated without disturbing the pellet. The pellet will be resuspended in 25 ml of 0.01 M of PBS and neutrophils counted using a hemocytometer.
To isolate PBMC from the blood, 10 ml of blood will be added to twenty five millilitres of 0.01 M PBS (warmed to RT) (Sigma-Aldrich, USA). The diluted blood will then be layered onto fifteen millilitres of histopaque (warmed to RT) (Sigma-Aldrich, USA). A density gradient will be formed which will allow the cells to separate. The layered cells will be centrifuged at 800 x g for 30 minutes with the centrifuge setting of deceleration off and slow acceleration. Following centrifugation, the whitish layer (buffy layer) will be aspirated to a new 50 ml tube and topped up with PBS and centrifuged at 300 x g for 10 min, with acceleration and deceleration at maximum. The supernatant will be discarded, and the cells will be resuspended in PBS before being counted. The cells will be centrifuged at 300 x g for 10 min with maximum acceleration and deceleration. Freeze media will be prepared by adding 500 µl of dimethyl sulfoxide (DMSO) (Sigma-Aldrich, USA) to four and half millilitres of fetal calf serum (FCS) (Sigma-Aldrich, USA). The supernatant will be removed, and the cells will be stored in 1 ml of freeze media at a cell concentration of 10 million cells per ml in a cryovial labelled with the PID and date and stored in liquid nitrogen. Plasma will be isolated by transferring ten millilitres of blood to a tube, which will be centrifuged at 1000 x g for 10 minutes, with maximum acceleration and with deceleration off. Ten cryovials labelled with the PID and date, are used to store plasma at -80°C at a volume of 500 µl.
All surfaces and pipettes will be decontaminated with RNAse away before RNA isolation will be done. RNA will be extracted using the PAXgene kit and the corresponding protocol. Briefly, blood from TB, LTBI and healthy participants will be collected in PAXgene tubes which contain anticoagulant to ensure the stability of the ribonucleic acid (RNA). The blood will be stored at 18–25°C during transit and upon arrival will be stored at -20°C at an angle on a wired rack for 24 hours before being transferred to the -80°C freezer. The frozen blood in the PAXgene tubes will be thawed in a water bath at 22–23°C for 20 minutes and the PAXgene tube will be inverted 8–10 times to before RNA isolation proceeded. Once thawing and inverting of the tube is complete, the PAXgene tube will be left at room temperature (RT) for a further 2 hours to ensure complete cell lysis. RNA will be isolated from the blood using the PAXgene kit according to the manufacturer’s instructions (PreAnalytiX, Hombrechtikon, Switzerland).
To determine the RNA quality, we will be using a Nanodrop Lite Spectrophotometer (Thermofisher™, Switzerland) and the absorbance will be read at 260 nm. The sample will be diluted (dilution factor 2) with 10 mM of Tris-hydrochloride, pH 7.5. The relationship between the absorbance and the RNA yield will be taken as A260 of 1 is equal to 44 ug/ml, according to the manufacturer’s instructions.
The iScript cDNA synthesis kit will be used for cDNA synthesis as per manufacturer’s instructions (Biorad, California, USA). The 5 x iScript reaction mix will be left to thaw at room temperature. The reaction will be set-up in sterile PCR tubes as follows; 500 ng of RNA will be added to each tube, followed by 4 µl of 5x iScript reaction mix, 20 µl of RNase free water and 1 µl of iScript reverse transcriptase. The total reaction mix will be 20 µl per tube. The tubes will be placed in a PCR machine and run on the thermocycler (Biorad, California, USA) using the following runs, 5 min at 25°C, 30 min at 42°C, 5 min at 85°C and on hold at 4°C. The resultant cDNA will be diluted 10x by adding 1 µl of cDNA to 9 µl of nuclease free water.
Qualitative polymerase chain reaction (qPCR) will be done on candidate genes as per the manufacturer’s instruction to determine the expression of the candidate genes in TB, LTBI and healthy participants. Briefly, half a microlitre of reverse primer, half a microlitre of the forward primer, three microlitres of nuclease free water and five microlitres of iTaq Universal SYBR green supermix (Biorad, California, USA) will be added to each well. A volume of nine microlitres of the mastermix will be added to each well. One microlitre of the diluted sample will be added to each well. The plate will be run on the Biorad CFX 96 thermocycler (Biorad, California, USA) according to the following protocol, 30 sec at 95°C, 5 sec at 95°C, 30 sec at 59°C, for 39 cycles. The melt curve analysis will be done at 65–95°C at 0.5°C increments.
A NETosis imaging kit (Cayman Chemical, Michigan, USA) will be used to quantify the amount of NETosis occurring in TB patients (n=4) compared to healthy participants (n=4). The assay will be performed as per manufacturer’s instructions. Briefly, 6 x 106 neutrophils isolated on the same day, will be transferred to a clean tube and adjusted in 0.01 M PBS to 1 x 106 cells/ml. A volume of six microlitres of Permeable Nuclear Red Reagent (5 mM) will be added to each well and incubated in the dark at 37°C for 15–30 minutes. A volume of 25 ml of PBS will be added to the cells, centrifuged at 250 x g and the supernatant aspirated. The cells will be resuspended in twelve microlitres (0.5 x 106 cells/ml) of NETosis Imaging buffer (1x). A volume of twelve microlitres of Extracellular Nuclear Green Reagent (5 mM) will be added to the tube and mixed before the cells are seeded into the wells at 100 µl/well in a flat-bottom 96 well plate (Greiner Costar, Sigma-Aldrich, Germany). A volume of 100 µl of stimuli or NETosis Imaging Buffer (1x) will be added to each well for a final volume of 200 µl. The plate will be centrifuged at 250 x g to pellet the cells at the bottom of the plate. The BioTek cytation 5 (Biotek, Vermont, USA) which uses brightfield and fluorescence microscopy will be used to image activity every 30 min-1 hour for six to twelve hours at a temperature of 37°C and 5% CO2. Green fluorescence indicates NETosis activity and red fluorescence is a marker for the nucleus (Figure 3). The neutrophils will be treated with optimized concentrations of various NETosis inhibitors depending on the chosen gene targets.
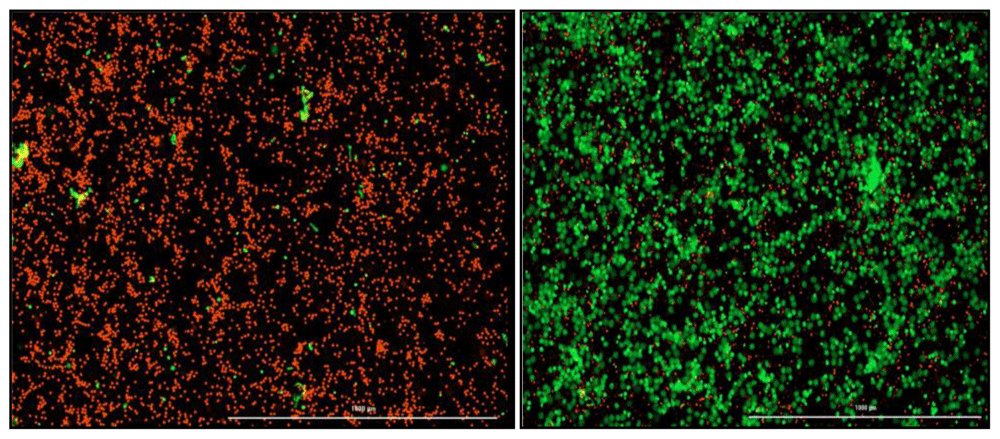
Red indicates intact neutrophils. Green indicates neutrophils undergoing NETosis and extruding their cellular DNA. (Left) Negative control which is only healthy neutrophils with no stimulation. (Right) Positive control, PMA stimulated neutrophils.
Student t-tests will be used to analyse the difference in gene expression between healthy, LTBI and TB participants. The data will be reported with the median and interquartile range. Pearson R Correlation or a Spearman R Correlation analysis will be done to determine if there is an association between protein and gene expression of specific NETosis associated protein and genes. All data will be analysed using Graphpad Prism v 9 software.
Proteomic analysis of lung tissue laser microdissections of various granulomas showed that proteins associated with neutrophils were abundant in necrotic regions of caseous and cavitary granulomas15. This indicated that neutrophils potentially contribute to necrotic damage observed in cavitary granulomas, due to their presence in these regions. This provides a potential link of the neutrophils with increased TB pathogenesis, hence the need to investigate their role during TB as potential target for therapy or as biomarkers. Identifying factors or mechanisms that contribute to lung tissue damage is vital in identifying markers of disease progression.
Neutrophils have been identified in the regions that border necrotizing tissue in other diseases17 and whether this is true for TB infection and granulomas remains to be determined. Recent studies have also identified the role of DNA-MPO11,16 or histones complexes10,18, ROS pathways9 and neutrophil elastase19 in tissue damage associated with various respiratory infections and as markers of disease progression. Studies in C3HeB/FeJ mice infected with Mtb have also shown that neutrophils contribute to necrosis and liquefaction of granulomas mediated by ROS events20. These studies suggest that similar mechanisms mediated by neutrophil derived proteins and cell death activities may be driving TB lung pathology in humans.
NETosis proteins have been detected in the serum of individuals with acute respiratory distress syndrome associated pathology (as reviewed by 21). However, whether NETosis directly contributes to damaging pathology observed in TB granulomas is not well known. To validate this, our initial aim is to establish whether NETosis associated genes are present in the blood and are differentially expressed in LTBI, TB and healthy participants. In addition, we aim to determine whether expression of specific genes associated with NETosis correlates with the spectrum of disease observed during TB infection (as reviewed by 22,23). In addition, these unique gene expression profiles may be used to identify potential biomarkers of disease progression that may be used to diagnose TB at point of care.
Ongoing clinical trials are investigating the use of NETosis specific gene inhibitors to reduce tissue damage associated with NETotic events24–28. Therefore, identifying potential targets for HDTs that may serve as adjunct therapies to current antibiotic regimens remains vital in combating the disease.
Due to the heavy TB disease burden in KZN, the study participants that will be enrolled in this study will provide important and relevant information regarding the tissue destruction mechanism that contributes to disease progression. A limitation of this study is that we will not be able to obtain matched blood samples from lung tissue donors. Another limitation of this study is that when performing the NETosis experiments it is difficult to distinguish between LTBI volunteers and those who are healthy in a timeous manner. This limits the ability of us to obtain a healthy blood volunteer to have an appropriate control.
The aim of this study is to identify potential biomarkers of TB disease progression that can lead to better diagnosis and ultimately, treatment of TB. We hope to characterize drivers of neutrophil mediated damage by developing a neutrophil in-vitro infection model. These findings may contribute to a better understanding of the neutrophil driven mechanisms that lead to damage often observed in the lungs of individuals with TB disease.
Data will be stored and available through the Africa Health Research Institute according to the institutes protocols which insure participant privacy and rights protection.
KLF and MJM wrote the manuscript. MJM conceived the study. KLF optimized the protocol and performed experiments. KRB, DM and KLF were responsible for developing and optimizing protocols for this study. TM provided helpful discussion in data analysis. DR assisted with instrumentation and imaging. MJM and TN supervised KLF and obtained funding for the study. All authors read and approved the manuscript in its current version.
We would like to thank all the study participants for their time and commitment to this study. We also thank the nurses and staff members and other personnel at clinical sites and all staff members at the clinical core at Africa Health Research Institute who helped with patient recruitment and sample processing.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: TB Pathogenesis
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology of tuberculosis and COVID-19 and Echinococcosis.
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: TB Pathogenesis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 29 Apr 22 |
read | |
|
Version 1 07 Dec 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)