Keywords
Mastitis, somatic cell count, California Mastitis test, reagent, sensitivity, specificity
This article is included in the AgriKnowledge gateway.
Mastitis, somatic cell count, California Mastitis test, reagent, sensitivity, specificity
Mastitis is regarded globally as the most common infectious disease in dairy cattle, as well as the most economically important (Halasa et al., 2007; Makolo et al., 2019; Shittu et al., 2012; Suleiman et al., 2013). The disease can be both clinical and subclinical in nature. Clinical mastitis can be visibly diagnosed and treated on routine physical examination, while subclinical mastitis often remains undetected (Reddy et al., 2014). An array of causative pathogens can be involved, entering the teat canal and multiplying in the udder, however most cases of mastitis are caused by a small group of bacteria including Staphylococcus aureus, Streptococcus uberis, Escherichia coli and Mycoplasma species (Carrillo-Casas & Miranda-Morales, 2012).
Due to the infectious nature of mastitis-causing pathogens, it is important for the livestock keeper to detect cases of subclinical mastitis in order to maximize cow health and well-being, as well as maintaining herd health (Kandeel et al., 2018). Subclinical cases of mastitis tend to show no visible changes in udder or milk, however the production of milk reduces and its composition changes, characterised by high somatic cell counts (SCCs) (Erskine, 2001).
SCC has been used as an indicator of mastitis in dairy herds since the 1960s (Pyörälä, 2003). Somatic cells contribute to natural defence mechanisms and include lymphocytes, macrophages, polymorphonuclear and epithelial cells (Pillai et al., 2001) and as such, these cells indicate an inflammatory process in intramammary infection. Counting somatic cells is used to distinguish between infected and uninfected quarters (Carrillo-Casas & Miranda-Morales, 2012), and has long been considered the gold standard in mastitis detection (Duarte et al., 2015). The California Mastitis Test (CMT) is an inexpensive, easily-applicable, cow-side test that enables the subjective assessment of the number of somatic cells present in a milk sample, as an estimation of the probability and severity of intramammary infection (Schalm & Noorlander, 1957).
The CMT involves combining equal volumes (2–3 ml) of milk and testing reagent, generally the sodium or potassium salts of long chain fatty acids, alkyl sulphates, alkyl sulphonates, alkyl arylsulphonates or alkyl arylsulphates (Leach et al., 2008). The gentle agitation of milk with these anionic-surface-active agents within a CMT paddle causes lysis of somatic cells and the release of cellular DNA. The DNA then agglutinates, giving the sample varying degrees of a ‘slimy’ or mucoid appearance depending on the number of cells within the sample. The extent of the reaction increases with the SCC of the milk (Leach et al., 2008). The degree of visible agglutination can be subjectively scored based on an ordinal scale and used as a qualitative test to estimate the SCC of milk samples (Moroni et al., 2018). Sensitivity, specificity and positive predictive values are important test parameters to assess test accuracy, validity and to minimize error, and are calculated at varying thresholds (Schepers et al., 1997; Thrusfield, 2018a).
In Ethiopia and Nigeria, dairy cattle are essential contributors to the agricultural industry and national economy, yet the productivity and profitability of the dairy subsector remains below potential in both countries (Abebe et al., 2016; Ameh et al., 1999; Shittu et al., 2012; Tangka et al., 2002).
Economic losses to the dairy industry due to mastitis include reduced milk quality and quantity, veterinary costs, culling and deaths (Degraves & Fetrow, 1993; Seegers et al., 2003). Subclinical mastitis is considered the most damaging and expensive due to the challenge in early detection (Adamu et al., 2020) although studies on the economic impact of mastitis in both Ethiopia and Nigeria are lacking (Adamu et al., 2020; Beyene & Tolosa, 2017; FAO, 2014; Mekonnen et al., 2019; Moru et al., 2018).
Commercial CMT reagents are not easily sourced in either Ethiopia or Nigeria, and the cost of importation limits the cost-benefit ratio in identifying and treating mastitis. However, as a domestic detergent-based CMT reagent is accepted in the UK as a cost-effective alternative (Leach et al., 2008), this project aims to investigate whether domestic detergents available in Ethiopia and Nigeria can also be utilised in the CMT, in place of the UK commercial CMT reagent, to achieve comparable results. This would thereby provide a cost-effective, widely available means of screening for mastitis in both Ethiopia and Nigeria, allowing for targeted treatment and disease control. In turn, this will decrease disease prevalence and production loss, increase herd health and welfare, and maximize milk quantity, quality, and economic value.
The milk samples came from Holstein Friesian cattle, with the majority of samples with high SCC (>400,000) originating from a farm in Medan Vale, Nottinghamshire, based on the known high number of mastitis cases and their willingness to engage with research projects. Other samples were collected from the dairy farm operated by the University of Bristol, based on ease of collection during COVID-19 restrictions. Samples were predominantly collected by veterinarians using aseptic technique (cleaning the teat, wearing gloves and discarding foremilk) (SEBI, 2019), with a few (approximately 20) collected by the respective farmers.
Samples were collected between August 2020 and February 2021. All samples collected for the purpose of California Mastitis Testing were collected in sterile 50 ml sample pots provided by Scarsdale veterinary practice, Derby, were stored at room temperature and tested within 48 hours of collection. All samples collected for the purpose of SCC analysis were collected in sterile 30 ml QMMS sample pots containing a milk preservative and posted within 48 hours of collection. Samples were identified with numbers in chronological order of collection so as to maintain blinding of details such as sample location. Two of the three operators in the validation study were blind to sample location and number as well as CMT reagents used. All operators were inexperienced in regards to performing the CMT prior to the study, and were trained using educational videos produced by SEBI (2019). All laboratory SCC results were sent to the respective veterinarians and farmers.
An accepted UK detergent-based CMT fluid is comprised of 40 ml Fairy liquid detergent (Proctor & Gamble), diluted with 160 ml water, with 1 ml of dark food colouring to enhance visualisation (Leach et al., 2008). As one cannot assume the domestic detergent available in Ethiopia and Nigeria will have an equal concentration of the anionic-surface-active agent required to lyse inflammatory cells in milk, the study first undertook a series dilution test to determine the most effective concentration of each detergent for visualising high SCC (>400,000 cells per ml milk1) and achieving the most comparable results to the UK CMT.
The study used six commercially available domestic detergents from Ethiopia (Reagents 1–3) and Nigeria (Reagents 4–6), and created five different dilutions: 40 ml detergent to 160 ml water; 50 ml detergent to 150 ml water, 60 ml detergent to 140 ml water; 70 ml detergent to 130 ml water; and 80 ml detergent to 120 ml water. Each dilution had the addition of 1 ml of commercially available food colouring from Ethiopia/Nigeria, depending on the detergent country of origin, to enhance visibility.
The five dilutions for each of the six detergents were then tested with 20 quarter milk samples. All samples were screened for inclusion in the study using the commercial UK CMT to ensure 15 samples with known high SCC values (CMT score 2–3) were included as well as five samples with known low SCC (CMT score 0–1) to test for false positives. Scores were based on reaction descriptions shown in Table 1.
To verify that the Ethiopian and Nigerian domestic detergent-based CMT reagents reliably indicate a high SCC (>400,000 somatic cells per ml milk), indicative of mastitis, each reagent was tested with a further 132 quarter milk samples. These samples were screened using the UK CMT upon entry into the study to ensure a range of CMT scores (0, 1, 2 and 3) were present in the validation study.
An additional two operators also tested the 132 samples using each of the six detergent-based CMT reagents, as well as the UK CMT. Both operators were blind to the origin of each reagent so as to reduce bias. Additionally, all samples were submitted for a laboratory SCC reading to confirm the accuracy of the CMT as a diagnostic tool for indicating high SCC samples.
The study identifies the point of significance as 400,000 cells per ml, determined by the laboratory SCC. Samples with SCC >400,000 were determined as a positive indicator of mastitis and thus we can expect a CMT score of 2–3, and samples <400,000 were deemed as weakly positive to negative, and thus we can expect a score of 0–1 on the CMT.
Validity of the CMT results using different detergents was assessed by comparing test sensitivity and specificity. Sensitivity is the proportion of animals that test positive that truly have the disease, while specificity is the proportion of animals that test negative that indeed do not have the disease. The results of each CMT were validated against a SCC, and sensitivity and specificity were calculated using the formula in Table 2 (Thrusfield, 2018b). High sensitivity minimises the number of false-negative results, while high specificity minimises the number of false-positive results. Confidence intervals were calculated using Wilson’s method (Thrusfield, 2018a)
| Test result | True status | |
|---|---|---|
| Positive | Negative | |
| Positive | a | b |
| Negative | c | d |
| Calculations | ||
| Sensitivity | a / (a + c) | |
| Specificity | d / (d + b) | |
| Positive predictive value | a / (a + b) | |
| Negative predictive value | d / (c + d) | |
Positive and negative predictive values were also assessed (Table 2, Thrusfield, 2018a). These take into account disease prevalence to show the probability of a test detecting a positive, or negative, animal. All calculations were performed in Microsoft Excel (Version 2108).
Individual detergents have differing concentrations of anionic-surface-active agent, and therefore, different detergents have varying optimal dilutions to achieve results most comparable to the UK CMT (Figure 1) (Vance, 2021).
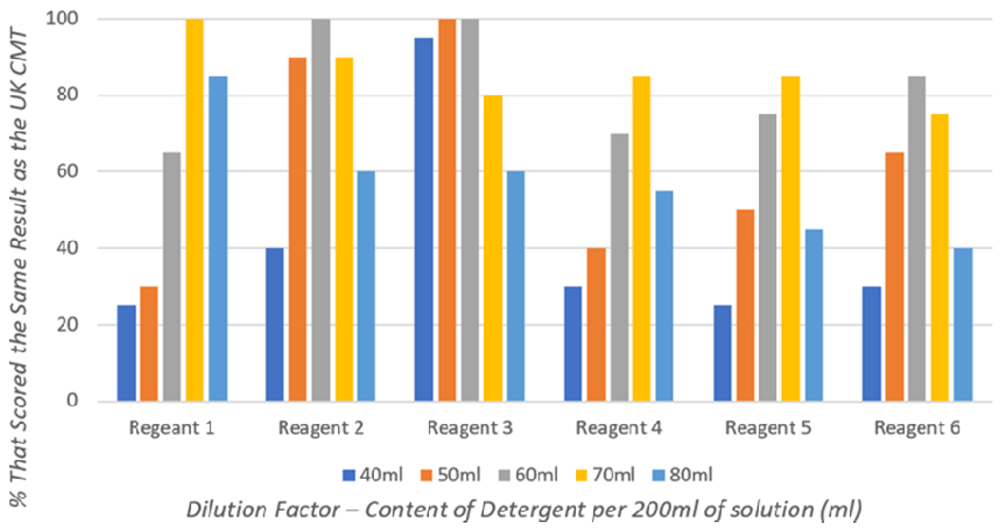
Reagents 1–3 (Figure 1), made with commercially available Ethiopian detergents, achieved 100% agreement with UK CMT results at varying dilutions: 70 ml detergent per 200 ml solution for Reagent 1; 60 ml per 200 ml for Reagent 2; and both 50 ml and 60 ml per 200 ml, in which this study takes an average of 55 ml per 200 ml, for Reagent 3.
Reagents 4–6 (Figure 1), made with commercially available Nigerian detergents, are seen to be less efficacious, with none achieving 100% equivalent results to the UK CMT. However, there are clearly optimal dilutions that achieve results most comparable to the UK CMT: 70 ml detergent per 200 ml solution for Reagents 4 and 5; and 60 ml per 200 ml for Reagent 6.
Results obtained from the dilution study are summarised in Table 3, demonstrating the optimum dilution of each detergent selected for the validation study.
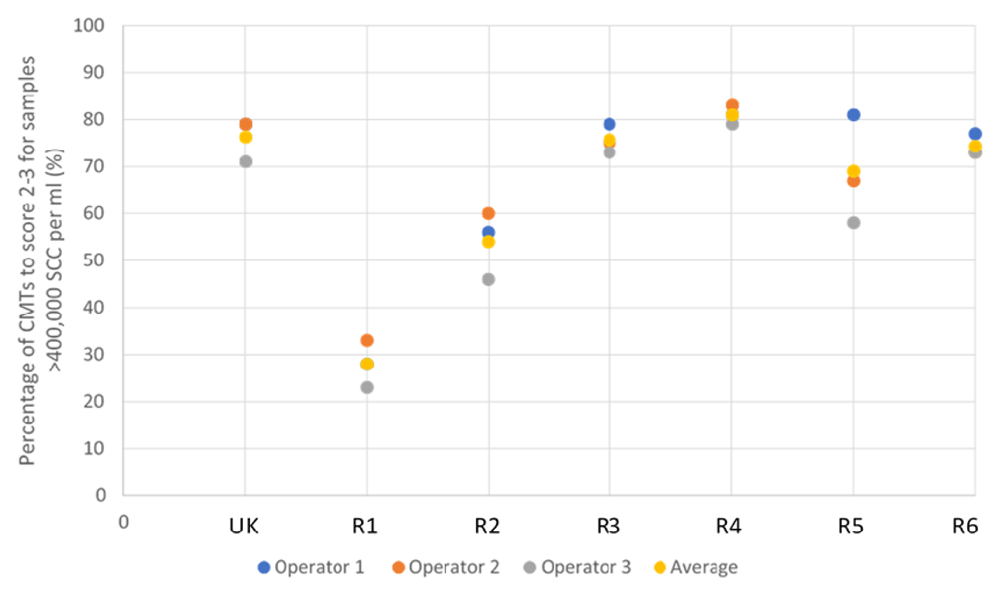
In the validation study, the majority of reagents scored similarly to the UK CMT, demonstrating greater than 95% specificity (Figure 2). Reagent 3, however, was a notable exception, scoring lowest, with an average of 84.2% between the three operators. This result, however, is still an acceptable level of specificity.

‘UK’ indicates UK commercial CMT reagent; ‘R’ indicates reagent.
Reagents were tested for sensitivity, by establishing the percentage of high SCC (>400,000) samples each reagent correctly identified as score 2–3 (Figure 3). It was observed that detergent-based CMT reagents have a higher specificity than sensitivity, and as such are more able to identify low SCC samples. It was, therefore, possible to best differentiate test validity of different detergent-based reagents by their ability to identify high SCC. Reagents 1 and 2 proved to be particularly poor reagents in the identification of samples >400,000 SCC, with 28.3% and 53.3% of samples correctly identified as high SCC, respectively. This is an insufficient proportion of high SCC samples correctly scored, due to the high number of false negative results. With the UK CMT indicating 76.3% of high SCC samples as score 2–3, Reagents 3 and 4 proved to be of similar efficacy, achieving 75.7% and 81.0%, respectively. Reagents 5 and 6 achieved slightly lower sensitivities with 68.7% and 74.3% high SCC samples identified as score 2–3, respectively.
‘UK’ indicates UK commercial CMT reagent; ‘R’ indicates reagent.
The percentage of detergent-based CMTs that achieved the same interpretated results as the UK CMT, i.e., negative to weakly positive tests score 0–1, and positive to strongly positive tests score 2–3, were established (Figure 4). Reagent 2 of the Ethiopian detergents (R1-3) is shown to have the highest percentage of similarity to the UK commercial CMT reagent with an average of 74.0%, despite Reagent 3 demonstrating the greatest sensitivity (of the Ethiopian reagents) (Figure 3). Of the Nigerian reagents (R4-6), Reagent 4 is shown as achieving the greatest percentage of score similarity to the UK CMT reagent, with an average percentage of 73.3% (Figure 4).
It can be seen that the UK CMT itself is an imperfect diagnostic tool due to the subjective nature of scoring, achieving an average of 96.3% and 76.3% efficacy at identifying negative to weakly positive, and positive to strongly positive samples, respectively (demonstrated in Figure 2 and Figure 3). Nonetheless, it is accepted for use in the field as an indicator of mastitis and, therefore, alternative detergent-based reagents should be held to similar standards. This suggests, therefore, that a better measure of test validity is achieved through each reagent’s ability to identify high SCC, rather than achieving the same interpreted score as the UK CMT.

As well as establishing the sensitivity and specificity for each reagent, positive predictive value (PPV) and negative predictive value (NPV) at identifying high SCC (>400,000) samples for each of the detergent-based CMT reagents and the commercial UK CMT reagent were calculated (Table 4). Almost all reagents demonstrate a high specificity, greater than 95%, with slightly lower specificity for Reagent 4 at 93.8%, and Reagent 3 at 84.2%. Greater differences in sensitivity were observed, ranging from 28.2% for Reagent 1, to 80.8% for Reagent 4. This is reflected in the PPV and NPV, where again Reagent 3 is shown to have the lowest PPV, indicating increased false positives; and Reagents 1 and 2 showing lower NPV, or increased false negatives.
It would appear overall that Ethiopian detergents are less effective in performing the CMT. However, of the three Ethiopian detergents investigated, Reagent 3 is of highest sensitivity (75.6%, CI% 63.9 - 86.3) and NPV, with the fewest false negatives, and so can be considered the most reliable for detecting mastitis, and thus individual cows requiring treatment, in the Ethiopian setting. In contrast, all Nigerian commercially available domestic detergents (Reagents 4–6), are more efficacious at identifying high SCC milk samples in a detergent-based CMT, with highest sensitivity achieved by Reagent 4 (80.8%, CI% 68.1 – 89.2), which had the fewest false negatives.
The CMT is a common test to indicate infected quarters in cases of subclinical mastitis. Mastitis is a significant disease in both Ethiopia and Nigeria due to its high prevalence and damaging effects on cattle health and productivity. It is, therefore, important for Ethiopian and Nigerian livestock keepers to be able to identify intramammary infections in order to control disease, whether that be to treat, reject the milk, dry off or cull high SCC individuals (Kandeel et al., 2018).
Due to the absence of cell counting laboratories, the CMT offers an easy, inexpensive, cow-side test to indicate the presence of infection and subsequent high SCC. However, the cost of reagent importation limits the cost-benefit ratio of the UK Commercial CMT. As a domestic detergent-based CMT is accepted in the UK, this study has investigated the use of six detergents, from Ethiopia (Reagents 1–3) and Nigeria (Reagents 4–6) to determine the optimal dilution for use in the CMT, and to validate the sensitivity and specificity these achieve compared to the UK CMT.
All of the Ethiopian detergent-based CMT reagents (R1-3) proved to be less effective than the UK CMT, however Reagent 3 had the highest sensitivity (75.6%, CI% 63.9 - 86.3) and NPV (84%), and therefore, despite the lowest specificity and PPV, was considered the best at ruling out disease and identifying cows of likely high SCC to treat. Thus, the authors recommend the use of detergent ‘Shagan’ (Reagent 3), commercially available in Ethiopia, at a dilution of 55ml detergent to 145ml water, with 1ml of indicator, for use in the CMT as an alternative to the UK CMT.
All of the Nigerian detergent-based reagents (R4-6) proved to be more effective than the Ethiopian reagents at identifying high SCC in milk. Reagent 4 proved to have the highest sensitivity (80.8%, CI% 68.1 – 89.2), and NPV (88%), and therefore, the authors recommend the use of the detergent ‘Sunlight’ (Reagent 4), commercially available in Nigeria, at a dilution of 70ml detergent to 130ml water, with 1ml indicator, for use in the CMT to identify high SCC.
Availability and cost are both important factors in being able to purchase reagents. In selecting detergents for the study, national availability was a selection criterion, along with detergents being in the higher end of the market, as better-quality products had been found to be more effective by Leach et al. (2008). For the CMT, the required volume of test fluid per cow is 12ml (3 ml per quarter); one litre of test fluid would therefore be sufficient for 83 cows. At the time of writing, the recommended Ethiopian detergent ‘Shagan’ costs 92–138 Ethiopian Birr (ETB) (equivalent to 2.0–3.1 USD) for one litre. To make one litre of the CMT fluid at the recommended dilution, 275 ml of Shagan is required, equivalent to 25–38 ETB (0.6–0.8 USD), equating to 0.1 ETB per quarter (0.002 USD). The recommended Nigerian detergent ‘Sunlight’ costs 800–866 Nigerian Naira (NGN) (equivalent to 1.9-2.1 USD) for one litre. To make one litre of CMT fluid at the recommended dilution, 350 ml of Sunlight is required, equivalent to 280–303 NGN (0.6-0.7 USD). This equates to around 0.9 NGN per quarter (0.002 USD). Commercial reagent for the UK CMT costs between 6.9–8.3 USD for one litre. The recommended local detergents, therefore, cost around one tenth of the commercial product and are both readily available.
The study does carry some limitations. Undertaking the study during the COVID-19 pandemic, and ensuring relevant guidelines were adhered to, was an ongoing challenge. In particular, the collection and testing of milk samples proved difficult, with many farms not allowing external access, vet practices frequently only open for emergency cases, and the difficulty in multiple operators testing the samples due to social distancing. This meant the project was very stop-start and took much longer than initially anticipated. Ideally, all samples in the validation study would have been tested by the same three operators, for consistency and to minimise bias, however due to restrictions, this was not possible.
The CMT is subjective in nature, which allows room for bias as well as operator error. A laboratory SCC automatically counts cells within a sample, reducing operator error and removing bias, but it is not without an error margin. The prolonged retention of samples, postal disturbances, transport and delivery may all contribute to sample modification. Additionally, the SCC also does not take away the value of a clinical exam, as mastitis and other mammary disease may not, in the rare occasion, be reflected in high SCC. All samples in this study are taken from UK Holstein Friesians cows. This study, therefore, assumes that indigenous breeds native to Ethiopia and Nigeria, though managed differently, will respond similarly to the CMT.
The study identified areas for further research. Firstly, there may have been differences in the efficacy of detergent-based CMT reagents in relation to how long the reagent had been made and left standing; further research is needed to clarify this. Secondly, the Nigerian food colouring was not dark enough and limited visibility, therefore a darker UK food colouring was added to enable testing. Future studies should look at food colouring and indicator dye available in Nigeria for their suitability for use in the CMT.
This study supports the use of detergent-based CMTs to improve the health and wellbeing of Ethiopian and Nigerian cattle through the diagnosis and control of mastitis, thereby increasing production and quality of milk, and improving the economic value of the dairy subsector. The most suitable local detergent and dilution was established for both countries and are recommended for use by livestock keepers to aid cost-effective diagnosis of mastitis and importantly reduce herd-level prevalence. This research is contributing to a larger ongoing study into mastitis in both Ethiopia and Nigeria, and will be used to produce educational pamphlets and videos as part of outreach activities to further the promotion of the CMT for the control and management of mastitis.
Harvard Dataverse: Replication Data for: A study of the effectiveness of a detergent-based California mastitis test (CMT), using Ethiopian and Nigerian domestic detergents, for the detection of high somatic cell counts in milk and their reliability compared to the commercial UK CMT. https://doi.org/10.7910/DVN/PULPBI (Vance, 2021).
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
With special thanks to MSD Animal Health for the research bursary and veterinary student sponsorship; Scarsdale Vets, Derby, UK, for assistance in sample collection and equipment provision; and thanks to Dr Veronica Nwankpa, Addis Abba, for supplying Ethiopian detergent.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pestiviruses and infectious diseases in cattle. I am not research active at present and my background is predominantly clinical work and teaching/course adminstration.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Dohoo IR, Smith J, Andersen S, Kelton DF, et al.: Diagnosing intramammary infections: evaluation of definitions based on a single milk sample.J Dairy Sci. 2011; 94 (1): 250-61 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Dairy herd health; mastitis diagnostics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 17 May 23 |
read | read |
|
Version 1 20 Sep 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)