Keywords
COVID-19 vaccines, repurposing, non-specific vaccine effects, real world data, real world evidence
This article is included in the Coronavirus (COVID-19) collection.
COVID-19 vaccines, repurposing, non-specific vaccine effects, real world data, real world evidence
Novel therapeutic and vaccine development for coronavirus disease 2019 (COVID-19) has advanced at extraordinary speed and scale. Against all historical precedent, multiple products have progressed from discovery through emergency regulatory approval in less than one year. In addition to these novel discoveries, scientific efforts have also focused on assessing the arsenal of existing products to determine whether off-label use of products could impact COVID-19 acquisition, disease progression and mortality. Within weeks of the identification of COVID-19, myriad claims were made about the potential efficacy of approved products, which consequently provoked supply shortages of multiple essential drugs and the initiation of many uncoordinated clinical trials.
As early as March 2020, observational studies and opinion pieces pointed to the potential impact of existing, broadly implemented viral vaccines on overall COVID-19 epidemiological patterns (Aaby et al., 2020; Fidel & Noverr, 2020; Gold et al., 2020), and multiple clinical trials were initiated across the globe to test these hypotheses1. The claims built on existing but contested literature that has examined immunological theory, observational data and clinical trial evidence to determine whether certain vaccines have beneficial or deleterious effects on all-cause mortality. One side of the debate hypothesizes that viral vaccines such as Bacillus Calmette-Guerin vaccine (BCG), measles-containing vaccine (MCV), oral polio vaccine (OPV) and Rabies reduce mortality; that non-live vaccines such as inactivated polio vaccine (IPV) and Diphtheria, Tetanus, Pertussis (DTP) increase mortality; and that stronger findings in both directions are observed among females (Higgins et al., 2016; Jensen et al., 2016; Kandasamy et al., 2016; Messina et al., 2019; Pollard et al., 2017). The logic is that certain vaccines might induce cross-protective ‘trained’ innate immune responses for some period after vaccine administration, while the adaptive system builds the more specific response to the target immunogen (Netea et al., 2016). Heterologous protection against other viruses thus occurs via induction of interferons and activated natural killer cells. Vaccine components such as adjuvants are also hypothesized to induce other immunological effects among T cells, including potential epigenetic changes which impact cell responses to future pathogens (Goodridge et al., 2016; Pollard et al., 2017).
A 2014 SAGE review found that the evidence could not ‘exclude nor confirm the possibility of beneficial or deleterious non-specific immunological effects of the vaccines under study on all-cause mortality’, and that findings were not sufficient to warrant a policy change in recommendation (Higgins et al., 2016; SAGE, 2014). A follow-on systematic review examined the relevant clinical trials, cohort and case-control studies on the mortality effects of BCG, DTP and MCV, and again suggested that while there was observed evidence of impact on all-cause mortality, more study was needed to identify whether there was any real mechanistic link (Higgins et al., 2016). In particular, reviews were concerned about the quality of the studies and potential for bias (Higgins et al., 2016), and others have previously noted overall methodological issues in epidemiological studies of non-specific effects (Farrington et al., 2009; Fine & Chen, 1992). Multiple studies also point to the multiple health-seeking behavioral attributes associated with vaccination that are likely to confound overall mortality outcomes (Fireman et al., 2009; Pollard et al., 2017).
Amidst this debate, the possibility of cross-protective innate immunity offered by other vaccines led us to undertake analyses of real-world data for signals of non-specific heterologous vaccine efficacy (VE) against COVID-19. If such VE were found and determined to be substantial, it would be relevant as an ancillary public health intervention for controlling the COVID-19 pandemic through vaccine repurposing and off-label human use in a short-time frame. In the present work, we directed attention to electronic health record-derived real-world data on MMR and recombinant adjuvanted varicella-zoster vaccines received in U.S. adult populations within 5 years prior to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in 2020. Additionally, as outlined below, we undertook this analysis to demonstrate a research methodology for using real world evidence to test hypotheses about the potential off-label activity of vaccines. Thus, the observations and conclusions we draw in this article are not intended to be used for clinical or other treatment purposes, and consequently the authors and their sponsoring organizations disclaim any and all liability for such uses.
Measles, Mumps & Rubella Vaccine (MMR). One hypothesized mechanism of heterologous protection involves overlapping of vaccine epitopes between the Spike (S) glycoprotein of the SARS-CoV-2 virus, the Fusion (F1) glycoprotein of measles virus and the envelope (E1) glycoprotein of the rubella virus (Sidiq et al., 2020). MMR is a live attenuated vaccine indicated for prevention of measles, mumps, and rubella infection, marketed in the U.S. since 1971. MMR vaccination in adults is often required in connection with employment in hospitals, health services and clinics, schools, and other settings and roles having high contact with the public, especially children. In most instances, employers requiring MMR vaccination of employees is conditioned on test results showing low or undetectable titer of antibodies against measles, mumps, and rubella viruses.
Recombinant adjuvanted varicella-zoster vaccine. Another hypothesized protection mechanism involves induction of augmented adaptive immune responses through dendritic cell activation and innate immune cell recruitment with certain contemporary adjuvants such as Adjuvant System AS01, which is a liposome-based vaccine adjuvant containing 3-O-desacyl-4'-monophosphoryl lipid A (MPLA) and the saponin QS-21 (Didierlaurent et al., 2014).
Bacille Calmette-Guérin (BCG). Some groups (see e.g. Curtis et al., 2020; Escobar et al., 2020; Gupta, 2020; O’Neill & Netea, 2020) have hypothesized a COVID-protective effect for BCG vaccination. However, we were not able to assess the impact of BCG using our dataset, as BCG is not widely used in the U.S., and when indicated it is primarily administered as an adjuvant treatment in bladder cancer. We found that this usage it is associated with a variety of confounding factors that limit the relevance of data from a cohort of bladder cancer patients receiving BCG treatment towards decision-making for a general population.
Randomized control trials rightfully remain the gold standard in determining safety and efficacy of products for licensure and widespread use, and as noted above, multiple scholars have emphasized the need for further studies assessing the non-specific effect of vaccines, on all-cause mortality and now against COVID-19. However, clinical development is lengthy and costly, and during a global pandemic, there are limitations in financial and human resources and infrastructure, and trials ultimately compete for patients needed to reach an answer on a product’s value. In this context, real-world data can generate a set of evidence to help prioritize constrained clinical trial resources and test products with the highest chance of success, provided that adequate sample size and endpoint event counts are available and appropriate adjustments are undertaken to mitigate biases and confounding.
Real world data (RWD) has recently been advanced to improve the responsiveness of research and regulatory agencies to the needs of the public and policymakers. The 21st Century Cures Act (2015) attempted to address the limitations of interpreting tightly controlled RCTs by incorporating real-world evidence (RWE) into the drug-labeling process. Similar programs have been initiated in various countries around the world, and in multi-national consortia, including the International Conference on Harmonization. When RWD is analyzed rigorously and used to generate package of RWE, this evidence can be used for hypothesis testing that separates the signal from the noise in claims of existing product effectiveness against COVID-19. This can, in turn, help to inform clinical trial targets and designs, and monitor real-world effectiveness of products as they are introduced.
In this context, analyzing real-world data can help optimize trial resources by assessing outcomes among large datasets and identifying leads and dead-ends among the array of on-formulary products. To demonstrate this, we applied a novel statistic method to a real word database of health records in the United States to assess the potential impact of twenty-five vaccines to see which, if any, merited detailed analysis. Based on this initial triage, we reviewed two viral vaccines (measles, mumps and rubella [MMR] and a recombinant adjuvanted varicella-zoster vaccine) against COVID-19 in the United States. We assessed these vaccines for potential impact on COVID-19 disease prevention and case fatality.
We utilized a commercially available electronic health record (EHR) derived de-identified privacy-protected HIPAA-compliant protected health information (PHI)-free secondary-use assented real-world data (RWD) repository (Cerner Healthe DataLabs RWD™, Cerner Corporation, Kansas City, MO, USA). All data was fully anonymized and there was no direct human subject research, and we thus did not seek approval from an Institutional Review Board. The repository comprises records from 2010 to present, with over 500 million care-related records and 160 million distinct patients. Material is encrypted end-to-end and pre-cleaned upon arrival into a data warehouse. Each data element is timestamped, and multiple episodes for a single person are longitudinally tracked with an encrypted key. The instance of the Cerner RWD used for the present study contains longitudinally-linked health records for 100% of patients in the care of 90 U.S. institutions and their affiliated ambulatory care clinics and physician offices, accessed via a private cloud environment with Python and Pandas software and ANSI-standard SQL. The data cleaning, transformation and further analyses were performed with R 4.0.4 (The R Foundation) and RStudio 1.4.1106 (RStudio, PBC).
At the time of our analysis, there were 373,032 PCR-proven, distinct COVID-19 patients in the Cerner Healthe DataLabs RWD™ repository between 01-MAR-2020 and 31-DEC-2020 in 90 tenants (extended data, Table S1; McNair et al., 2021). We retained 347,570 records in the 38 tenants whose contributed cases comprise more than 0.5% of the aggregate sample size (extended data, Figure S1; McNair et al., 2021). Demographic characteristics of all patients are available in Table S1 (McNair et al., 2021).
After an initial review of 25 vaccines in common use in the US, we narrowed the analysis to two vaccines with signals warranting further attention: an MMR vaccine, and a recombinant adjuvanted varicella-zoster vaccine. For MMR-related analyses, we confined the records to persons aged 25 to 64 years old inclusive at the time of their 01-MAR-2020 and 31-DEC-2020 index episode (Table 1). In general, such subjects received MMR in connection with their employment as health care workers, first responders, teachers, and other professions in which employers require periodic titer measurement and revaccination with MMR, and the vast majority of vaccinated MMR subjects are female. Not surprisingly, there are relatively few prevalent persons aged 65 and older at the time of their 01-MAR-2020 and 31-DEC-2020 index COVID-19 episodes who received MMR vaccination within the preceding 5 years. In similar fashion, we confined our analyses related to the recombinant adjuvanted varicella-zoster vaccine just to persons 50 years of age or older and excluded subjects receiving that same vaccination at younger ages (Table 1).
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2; COVID-19=coronavirus disease 2019; PCR=polymerase chain reaction.
For each PCR-proven COVID-19 subject in the real-world data repository selected by SQL query inclusion-exclusion criteria, we utilized vaccination for influenza within the lookback time horizon as a proxy for vaccine non-hesitancy. This was done to account for potential confounding due to health-seeking behavioral attributes that might be associated with vaccination (Fireman et al., 2009; Pollard et al., 2017). We required ICD-10-CM U07.1 code denoting COVID-19 disease and a positive PCR result for SARS-CoV-2. We recognize that PCR sensitivity is less than 100% and this composite selection criterion may therefore be overly conservative. We designed SQL to retrieve records from the real-world data repository with selection criteria to provide comparability of cases and controls in terms of (a) a diverse range of comorbid ambulatory care-sensitive conditions (ACSCs) and (b) time span of longitudinal follow-up subsequent to the index episode, as well as likelihood and timing of contact with the health system enabling endpoint ascertainment. The subjects included in our analysis are patients whose EHR records showed that they received at least one medication prescription in connection with their care episodes. We recognize that this selection criterion excludes some people without known ACSCs who presented to an ambulatory clinic/office or who may have been dismissed with no prescribed medications or only with recommended OTC medications, and no follow-up plan.
Persons who presented to the institutions’ emergency departments or who were admitted to hospital as inpatients had, in general, clinical findings and physician orders indicative of more severe COVID-19 disease than persons whose care was delivered in ambulatory settings. Emergency Department patients and inpatients tended to have multiple symptoms, larger numbers of physician orders, larger numbers of episodes of care preceding the index episode, larger numbers of comorbid conditions, and laboratory result values in the records of the index episode consistent with severity of illness such as would require care in an acute-care venue. By contrast, records for many of the ambulatory outpatient episodes lacked any SNOMED-CT observation or finding denoting a COVID-19 symptom, despite having PCR-positive test results associated with the U07.1 ICD-10-CM code.
The magnitude of a treatment benefit, if one exists, may depend on the patient’s phenotypic and other characteristics. It is therefore important to investigate the heterogeneity and consistency of the treatment effect across subgroups to improve the reliability of study findings and their projectability to other populations. An observed heterogeneity in treatment effect across subgroups can arise because of chance alone, whereas true heterogeneity may be difficult to detect by standard statistical tests because of low power. For this reason, we measured heterogeneity across source institutions using the I2 statistic, with higher values reflecting increasing heterogeneity (Higgins & Thompson, 2002; Higgins et al., 2003; Lin et al., 2017). Sources of heterogeneity were assessed by subgroup analysis and by meta-regression (Baker et al., 2009; Higgins & Thompson, 2002; Van Houwelingen et al., 2002). Subgroup analyses included mean age and gender ratio by site.
Some recent papers (e.g. Gold et al., 2020) about possible protective effects of vaccines, including MMR, BCG, and varicella-zoster vaccines have done little to control for bias and confounding in observational data. By contrast, we recommend that propensity score adjustment, heterogeneity estimation, and meta-analytic average treatment effect (ATE) estimation are essential when performing hypothesis testing on real-world data. Using stepwise forward variable selection and the purposeful variable selection method of Bursac and coworkers (Bursac et al., 2008), we constructed covariable-based propensity scores [CBPS] (Fong et al., 2018; Imai & Ratkovic, 2014; Ning et al., 2020; Schneeweiss et al., 2009) and performed 1:4 matching vaccine-exposed cases to controls based on these scores.
On preliminary analysis of the prevalent PCR-proven COVID-19 cases in the real-world data (McNair et al., 2021), prior vaccinations with a recombinant adjuvanted varicella-zoster vaccine or MMR do not appear to have significant protective effects against SARS-CoV-2 infections (extended data, Table S3; McNair et al., 2021), but they do appear to show substantial effects reducing COVID-19 case fatality rate, with the MMR result rendered inconclusive when using additional statistical controls described below (extended data, Table S2; McNair et al., 2021). Because of the null result for a protective effect, for the subsequent analyses we focus on the effects of prior vaccinations in reducing COVID-19 case fatality rate. However, the case fatality ratio (CFR) when expressed as an effect size exhibits substantial heterogeneity among the institutions contributing the cases to be analyzed. I2 values indicate that 99% of the total variance in MMR- and recombinant adjuvanted varicella-zoster vaccine-associated mortality-prevention effect-size across studies is due to systematic (site-wise by source institution) heterogeneity rather than due to random sampling error. Bias of I2 estimates is expected to be negligible for a collection of 90 source institutions as in the Cerner repository of COVID-19 cases and contemporaneous populations of patients receiving care during the pandemic (von Hippel, 2015).
We determined the absence of disparities in vaccine provisioning by race and gender by the Cochran-Mantel-Haenszel test (Landis et al., 1978; Mantel, 1963; Mantel & Haenszel, 1959) noting that the exact version of the generalized Cochran-Mantel-Haenszel test does not necessarily result in the same p-value as Fisher's exact test (Davis, 1986). Evidence-combining was performed by standard methods of meta-analysis (Lipsey & Wilson, 2001; Singh et al., 2005; Van Houwelingen et al., 2002).
Our several attempts to use covariables available in the EHR-derived RWD repository to devise a suitable propensity score predicting MMR exposure were unsuccessful in achieving acceptable balance. Hosmer-Lemeshow calibration was poor across propensity score quartiles for MMR exposure. Furthermore, the best of our provisional propensity scores for MMR exposure demonstrated residual heterogeneity I2 = 43%, a value that we believe to be too large to permit reliable interpretation of analyses of efficacy endpoints in real-world data (ideally below 10%; see (Austin & Stuart, 2015; Berger et al., 2017)). The average treatment effect size calculated for the available data is not significant, and the sample size is not presently sufficient to power detection of so small an effect size (Munoz & Rosner, 1984; Nam, 1992; Wallenstein & Wittes, 1993; Woolson et al., 1986). For this reason, we plan to defer further analysis of MMR non-specific protective efficacy until a larger sample size has accrued or pooling of data and meta-analysis with other repositories (such as the Oxford University – OHDSI Scylla repository) becomes possible. In general, findings were consistent that the presence of MMR vaccination did not yield lower case fatality rates than controls, but given the limitations described above, we could not derive clinically significant results from this dataset.
By contrast to MMR, the available data on the recombinant adjuvanted varicella-zoster vaccine yielded a propensity score having acceptable performance in terms of covariable balance and reduction in heterogeneity after matching (Figure 1–Figure 4). Here, the overall odds ratio for CFR was 0.43 (95%CI: 0.38 - 0.48). Thus, the case fatality-related non-specific vaccine efficacy (VE) for a recombinant adjuvanted varicella-zoster vaccine vaccination prior to SARS-CoV-2 infection is approximately 100*(1.00 – 0.43) = 57%.
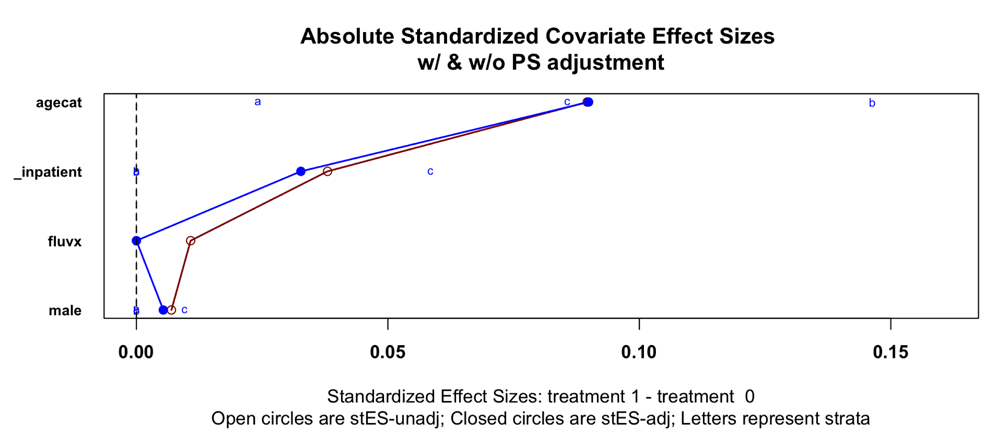
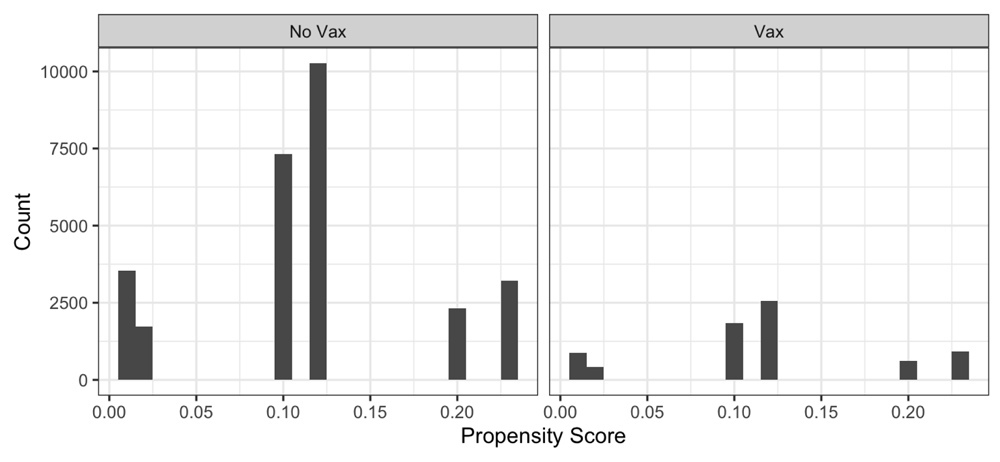
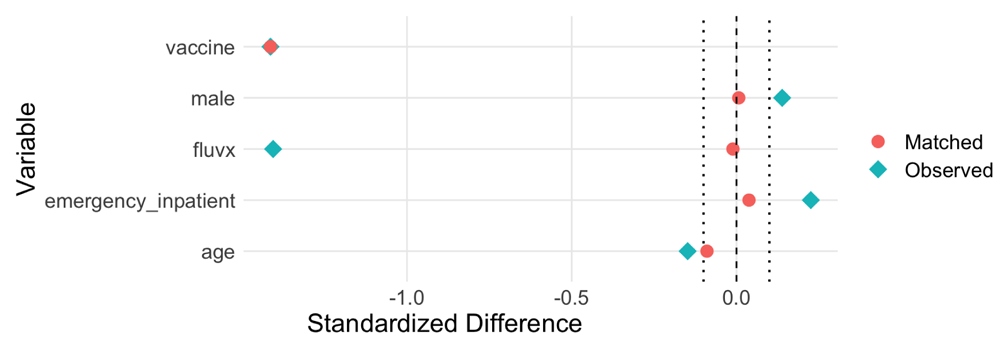
In the present real-world data analysis we did not find evidence that recent prior vaccination of adults with either a recombinant adjuvanted varicella-zoster vaccine vaccine or MMR vaccine conferred substantial protection of individuals against acquiring SARS-CoV-2 infection (extended data, Table S4; McNair et al., 2021). For case fatality rate, inadequate MMR sample size and covariable distributions prevented construction of a propensity score having adequate performance for weighting or matching on covariables to reduce bias and heterogeneity sufficiently to enable reliable analysis. Sample size and event counts were, however, sufficient for the cohort who received a recombinant adjuvanted varicella-zoster vaccine to enable construction of a suitable propensity score, and analysis of propensity score matched cases and controls revealed a clinically meaningful benefit in reduced case fatality rate.
Regarding limitations of the present analysis, we recognize that there may likely be a temporal relation of non-specific protection by vaccines against COVID-19 or other infections. We conducted exploratory analyses [using methods reported by (Zhang et al., 2020)] of the recency of recombinant adjuvanted varicella-zoster and MMR vaccinations to the date of presentation for the index COVID-19 episode. However, in the real-world data presently available for analysis there were insufficient patients exposed to a recombinant adjuvanted varicella-zoster vaccine between 2015 and 2017 to enable detailed analysis of the effect of the time interval between vaccination and SARS-CoV-2 infection. Therefore, our analysis relied on the recombinant adjuvanted varicella-zoster vaccine exposures between 2017 and 2019, inclusive. Further analysis of temporal relationships and durability of non-specific heterologous protection awaits the accrual of a larger cohort or pooling of data with other investigators’ real-world data repositories.
Beyond the concerns of the present work, we believe that this study illustrates the importance of certain best practices for reliable analysis of real-world data [see (Berger et al., 2017; Fang et al., 2020; Stürmer et al., 2020). In particular, we recommend the following six rules and practices:
Only retain those sites whose contributed cases comprise at least 0.5% of the aggregate sample size.
Perform propensity score adjustment to reduce bias and confounding.
Assess possible residual bias or disparities with the Cochran-Mantel-Haenszel test or other suitable approach.
Proceed with multivariable inferential or causal modeling only if the residual heterogeneity is less than 10% and the residual post-propensity score adjustment maximum standardized mean difference on the covariables is less than 0.10.
Perform meta-analysis to determine the average treatment effect (ATE) across the retained sites and examine forest plots and related outputs of meta-analysis.
Verify the statistical power afforded by the available sample size for measuring the modeled ATE.
The first of these practices reflects considerations of the difficulty of collecting real-world data involving a large number of mapped, curated variables for propensity score adjustment and inferential modeling. Typically, real-world data tend to consist of dozens or hundreds of variables but a smaller number of data-contributing sites, which means p > n (p is the number of predictor variables and n is the number of observed sites). In addition, exposure counts and outcome events often have over-disperse distributions and have low prevalence. Thus, the data are sparse and unbalanced. This makes it challenging to apply classical statistical analysis methods (Hastie et al., 2009). Under such conditions, a threshold is applied to focus on patterns and statistical associations within sites having substantial prevalent cases and controls, such that data sparsity will be less influential on analyses and analyses will not be dominated by one or two contributing sites having large numbers of prevalent cases. Minimum prevalence thresholding in RWD analysis is similar to the practice of applying sample selection filters for microbiome analyses of diversity based on minimum operational taxonomic unit (OTU) abundance and minimum total number of 16S rRNA reads (Bokulich et al., 2013).
In summary, speculation about possible non-specific heterologous vaccine efficacy likely can only be conclusively resolved by appropriate randomized control trials. However, valuable evidence may be obtained from real-world data, provided that adequate samples size and endpoint event counts are available and appropriate adjustments are undertaken to mitigate biases and confounding. Such evidence includes estimates of effect-size and average treatment effectiveness, which may help to inform statistical analysis plans for prospective trials (Levenson, 2020). While there are several theoretical hypotheses regarding the concept of heterologous trained immunity possibly conferred by live attenuated vaccines and vaccines with novel adjuvants, well-designed controlled studies are needed, before considering heterologous vaccination as a modality for preventing COVID-19 infection or mitigating its severity or outcomes.
Zenodo: Looking in the medicine cabinet: Methods for using real-world data to assess the impact of MMR and Recombinant adjuvanted varicella-zoster vaccine on COVID-19 prevention and case fatality. http://doi.org/10.5281/zenodo.5093688 (McNair et al., 2021).
This project contains the following underlying data:
- 03_COVID_all_cleaned.csv (CSV file with all COVID+ subjects in Cerner institutions)
- 04_COVID_mmr_data_cleaned.csv (CSV file with COVID+ patients between 25–64 years old, including institution id, age category, gender, whether patient is in emergency department or inpatient, flu vaccine history, MMR vaccine history and mortality outcomes)
- 05_COVID_mmr_data_matched.csv (CSV file matching MMR vaccine-exposed cases to controls based on propensity scores)
- 06_COVID_zoster_data_cleaned.csv (CSV file with COVID+ patients above 50 years old, including institution id, age category, gender, whether patient is in emergency department or inpatient, flu vaccine history, zoster vaccine history and mortality outcomes)
- 07_COVID_zoster_data_matched.csv (CSV file matching zoster vaccine-exposed cases to controls based on propensity scores)
- 08_General_25_64_data_cleaned.csv (CSV file, all patients in Cerner institutions between 25 – 64 years old, including institution id, age category, gender, whether patient is in emergency department or inpatient, flu vaccine history, MMR vaccine history, SARS-CoV-2 infection and COVID-19 mortality outcomes).
- 09_General_over50_data_cleaned.csv (CSV file, all patients in Cerner institutions above 50 years old, including institution id, age category, gender, whether patient is in emergency department or inpatient, flu vaccine history, zoster vaccine history, SARS-CoV-2 infection and COVID-19 mortality outcomes).
- 10_Included_tenants.csv (CSV file, institution IDs whose contributed cases comprise at least 0.5% of the aggregate sample size).
Zenodo: Looking in the medicine cabinet: Methods for using real-world data to assess the impact of MMR and Recombinant adjuvanted varicella-zoster vaccine on COVID-19 prevention and case fatality. http://doi.org/10.5281/zenodo.5093688 (McNair et al., 2021).
This project contains the following extended data:
- 01_Basic_Analysis.R (descriptive analysis script in R, for use with cleaned data files 3, 4 and 6)
- 02_Cleaning.R (Script demonstrating how Cerner data was cleaned upon download)
- 11_MMR_ps.R (R script to run for MMR-related files analysis)
- 12_Zoster_ps.R (R script to run for zoster-related files analysis)
- S1_20210712_vx_off_target_pubdraft_supplemental.docx (Tables, Graphs and scripts associated with publication)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
No
Are sufficient details provided to allow replication of the method development and its use by others?
No
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Heterologous vaccine protection against COVID-19. Immunology. Medical pathophysiology. R. Data analysis
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
No
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vaccine impact, respiratory viral disease, infectious disease epidmeiology
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
Yes
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Public health, programming, monitoring, epidemiology
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vaccinology, Immunology, Immunopharmacology
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
No
Are sufficient details provided to allow replication of the method development and its use by others?
No
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Observational research, machine learning, epidemiology, health informatics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 1 30 Jul 21 |
read | read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)