Keywords
Laboratory, research capacity strengthening, good laboratory practice, insecticide, test facility, quality management system, quality management systems, capacity strengthening
Laboratory, research capacity strengthening, good laboratory practice, insecticide, test facility, quality management system, quality management systems, capacity strengthening
Building research capacity in public health and related fields is essential to the generation of robust, innovative and locally relevant scientific data. When research staff are highly skilled and research infrastructure at institutions is strong, the evidence generated by these institutions can inform national policies, support progress towards population health goals and contribute to socioeconomic development1–4. Research capacity strengthening is increasingly an area of focus for international development and global health partners and funding bodies5,6. With increasing investment of funds to support research capacity strengthening, there comes an increased need to evaluate the impact of this investment on data quality7. Test facilities are a key component of national research capacity. Attention is commonly focused on clinical diagnostic and research facilities, their role in diagnosis and support in disease and epidemiological surveys8. However, non-clinical and basic science facilities also have key roles to play in global health research9. This can include supporting entomological mapping surveys such as insecticide resistance mapping, generating scientific evidence that can inform the discovery of novel compounds for therapies, development of new products that may have uses in public health, including the control of vectors of diseases, and assessing the safety of these compounds and products before they are used.
This study focuses on a research capacity strengthening project supporting seven test facilities in Africa towards full compliance with Organisation for Economic Co-operation and Development (OECD) principles of Good Laboratory Practice (GLP)10. These test facilities are all engaged in the evaluation of mosquito vector control products, including long-lasting insecticidal nets and indoor residual spraying formulations11. Each test facility consists of an insecticide testing facility (ITF), a molecular biology laboratory, experimental hut sites, an insectary, and animal houses. Data generated by these test facilities inform decision making at a national and international level, as these test facilities have historically conducted laboratory and field efficacy trials on vector control products for evaluation by the WHO Pesticide Evaluation Scheme (WHOPES)12 which supported national programmes and other stakeholders in the selection and safe and judicious use of public health pesticides. With ever-mounting challenges related to increasing insecticide resistance and changes in vector profile and distribution due to climate change, there is a pressing need for innovative vector control products, tools and approaches. To support this, WHO has now transitioned the function for evaluating these products to the WHO Pre-Qualification Vector Control Team (WHO PQ-VCT), to align the quality assurance of vector control products with existing prequalification processes within WHO13. Test facilities will now generate data on behalf of companies for the evaluation and prequalified listing of vector control products by WHO PQ-VCT, which guides UN agencies, other international organizations and country-level procurement bodies on the procurement of products for malaria management and eradication14. Whilst test facilities are moving towards GLP certification, WHO PQ-VCT can inspect data-generating facilities to ensure quality data. However, once sufficient test facilities have been granted GLP certification, WHO PQ-VCT will require companies ‘to develop a product dossier which includes data and information to support the safety, efficacy, and quality requirements appropriate to the product type and generated according to Good Laboratory Practices (GLP) and appropriate Quality Management System (QMS)’15. The conducted of studies compliant with GLP principles will ensure that data generated for product registration purposes are reliable, reproducible and auditable and will be recognised by scientists and regulatory authorities worldwide. Each test facility was supported towards GLP certification by the Innovative Vector Control Consortium (IVCC), with funding from the Bill & Melinda Gates Foundation being used to support the development and implementation of quality management systems, infrastructure improvements, facility inspections to identify and address nonconformances with GLP principles and staff training activities.
Research capacity strengthening has been defined as ‘a process by which individuals, organisations, and society develop the ability to perform [research] functions effectively, efficiently and in a sustainable manner to define objectives and priorities, build sustainable institutions and bring solutions to key national problems’16. This definition highlights that research capacity strengthening happens at three levels: the individual level, the organisational or institutional level, and the societal or national/international level. In capacity strengthening, initiatives are often focused at one of these three levels8,17, with programme goals and evaluation of programme success aligning directly with these levels. In this study, the described goal was at the institutional level – developing a QMS compliant with the principles of OECD GLP and being granted GLP certification. Despite an institutional-level goal, the interventions required to implement this system acted at individual, institutional, and national/international levels.
The purpose of this study was to capture both the primary effects of the GLP certification project on each institution’s research capacity, the secondary effects at the individual and institutional level, and any ripple effects beyond the research system. The relationships between effects at different levels are identified. These effects are compared to an existing framework for the evaluation of research capacity strengthening initiatives, to identify new areas for future laboratory capacity strengthening programmes to consider when developing and evaluating their interventions. In addition, we saw ripple effects of the project beyond research capacity strengthening for both individuals within each facility and into the community surrounding them.
Ethical approval to conduct this research study was obtained from the Liverpool School of Tropical Medicine Research Ethics Committee (approval number 18-041), the National Institute for Medical Research Tanzania (ref NIMR/HQ/R.8c/Vol./I/554), and the Centre Suisse de Recherches Scientifiques en Côte d'Ivoire Institute Review Board (ref 19-549). Institutions taking part remotely (i.e., interviews with members of research staff via Skype/email) provided an institutional approval document in lieu of in-country REC approval, as per point 3c of the LSTM’s Approval Processes for Network and Capacity Strengthening Studies.
Participants were informed about the research using participant information sheets18. Written consent was obtained from each participant prior to undertaking an interview.
Seven insecticide test facilities engaged in the testing of novel vector control products for the purpose of supporting malaria control programmes have received investment and support from IVCC to achieve GLP certification. Of these seven facilities, five have been included in this study, encompassing test facilities in Tanzania, Côte D’Ivoire and Burkina Faso. These five test facilities encompass a diverse array of contexts. PAMVERC-KCMUCo, Tanzania, provides crucial information on how GLP certification can be achieved, being the first insecticide testing facility in Africa to do so. Comparison between East and West African contexts was facilitated through inclusion of Centre Suisse de Recherches Scientifques en Côte D’Ivoire (CSRS) and Institut de Recherche en Sciences de la Santé (IRSS), Burkina Faso. Comparison between government and non-government test facilities was facilitated through inclusion of National institute For Medical Research (NIMR), Amani Centre, Tanzania and Ifakara Health Institute (IHI), Tanzania. These contrasting test facilities enhanced our ability to identify both direct and indirect effects of investments in developing a QMS. Generalisability of findings was assessed through using these facilities to compare effects of investment in QMS in a diverse range of contexts, including different national policy contexts and government/non-government supported test facilities.
To capture the views of individuals who had exposure to the GLP certification process at all levels of these test facilities, a maximum-variation purposive sampling strategy was used19. Sampling included those who hold key roles within a test facility, as determined by a case-study conducted on the first test facility to achieve GLP certification, KCMUCo-PAMVERC20, as well as multiple representatives at each organisational level of the facility. This allowed triangulation between different data sources to determine the trustworthiness of findings. Test facility organograms were used to identify relevant participants, with guidance from stakeholders at IVCC and GLP project managers.
Semi-structured interviews were conducted with individual staff members involved in the GLP process in three test facilities; KCMUCo-PAMVERC, NIMR Amani Centre, and CSRS. The interview topic guide18 was developed based on previous studies of laboratory capacity strengthening8, with additional questions derived from findings from a case study of the GLP certification process at PAMVERC-KCMUCo20. One overarching question was specifically related to perceived effects of the project. However, due to the semi-structured nature of the interview, interview participants reflected on the effect of the project throughout the interview. Specific questions asked from the topic guide were matched to the roles and responsibilities of the interviewee. Interviews were audio-recorded and transcribed in full. All interviews were conducted in person, in a private room or office, by two researchers, one of whom had a technical understanding of GLP requirements in insecticide testing facilities and the other having systems evaluation experience. Whilst the lead researcher spoke basic French and Swahili, for interview participants who preferred to undertake the interview in a language other than English, a trusted colleague or research student sat in on the interview to aid with translation.
A combination of email and remote video-call interviews were conducted with individual staff members involved in the GLP process at two other test facilities, IRSS and IHI. This was necessitated by restrictions on travel and reduced working hours following the COVID-19 pandemic, which resulted in significant disruption from March 2019. The overarching questions asked in these interviews were retained from the semi-structured interview guide used for in-person interviews. Follow-up questions, where relevant, were conducted via video-call or email.
A framework analysis21 was used to identify themes emerging from the interview transcripts following the five-step process of familiarization, identification of thematic framework, indexing, charting and mapping/interpretation. The framework identified was the Research Capacity Strengthening evaluation framework developed by Khisa et al., from African Population and Health Research Center, Nairobi, Kenya and Centre for Capacity Research, Liverpool School of Tropical Medicine, UK22. This framework delineates the identified and envisioned effect of research capacity strengthening initiatives at the individual, institutional, and national/international level, developed from a review of the research capacity strengthening literature and refined in consultation with research capacity strengthening funders, implementers, managers and evaluators (Table 1). Following familiarisation with the interview data, further themes were identified and incorporated into the framework, while retaining the individual, institution, and societal level structure. All interview transcripts were indexed using NVivo software version 11 (QSR International).
A total of 65 members of staff from five test facilities participated in this study. 66 were approached to take part, with one declining to take part. Of these staff, 16 were laboratory/insectary technicians or attendants, 17 were from non-scientific administration/information technology positions, 22 were from scientific middle-management positions, and 11 were from scientific senior management positions. 49 were male and 16 were female. Anonymised identifiers have been used for quotes from transcripts, highlighting the role of the interview participant but not the test facility they are connected to. These are presented in Table 2 and referenced by section in the text.
From the interviews, the research capacity strengthening effect of the programme at the institutional level was consistently identified. These primary effects were particularly evident in the research environment, both physical and administrative, sustainable provision of high-quality training, and the capacity of the test facility to deliver competitive research, i.e. GLP-compliant studies. There were also secondary effects identified at both the individual and national/international level. At the individual level these effects were related to the training delivered as part of the GLP project, but there was also a positive relationship between the strengthened research environment and individual level motivation and job satisfaction. At the national/international level networks between institutions were developed, which also had the effect of further strengthening individual test facilities (institutions) as inter-facility learning was made possible. This meant lessons from test facilities at more advanced stages in the process could be applied to those at earlier stages.
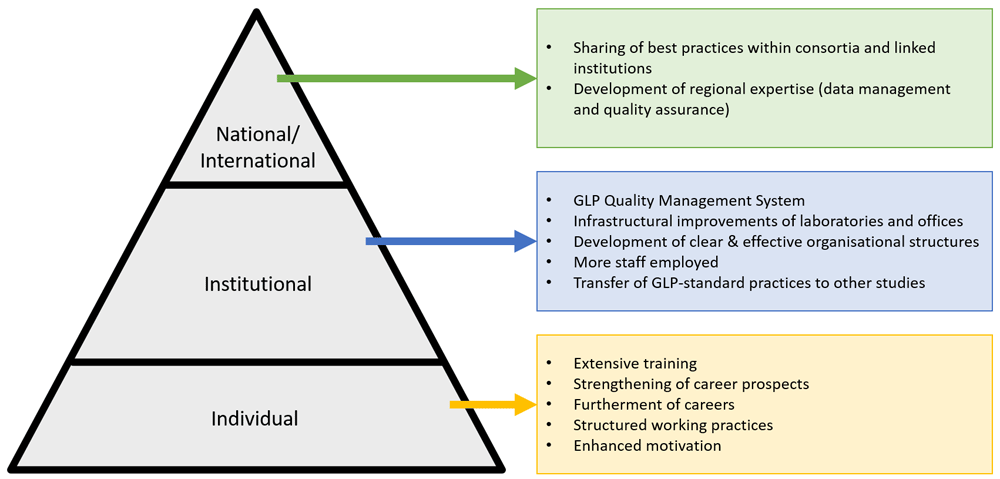
At the institutional level, the GLP quality management system, infrastructural improvements of laboratories and offices, development of clearer and more effective organisational structures, more staff employed, and the transfer of GLP-standard practices to other studies were all identified as research capacity strengthening effects resulting from the GLP project.
The development of a GLP-compliant quality management system and, at some test facilities, the achievement of GLP certification following inspection by the GLP monitoring authority SANAS, is a clear outcome of the work undertaken through the IVCC project. Of the seven test facilities included in the wider project, two have achieved GLP certification to date, and four have submitted their application for GLP certification to SANAS. As a result of GLP certification, these two test facilities were able to deliver national/internationally competitive research, with data meeting international standards. This effect extended also to non-GLP studies conducted at these test facilities, as best practice from GLP studies was applied also to non-GLP studies by both scientists involved in the GLP project and other scientists within the institution, particularly with respect to study documentation and use of Standard Operating Procedures (SOPs). Thus, the overall quality of data generated at these test facilities was enhanced. (Quotes: INS3) Test facilities also identified broader effects on working practices, resulting from the implementation of GLP standards. In particular, increased structure in working practices resulting in a range of benefits including cost savings on reagents, more effective problem solving, and better organisation of work throughout the test facility. (Quotes: INS5)
Career pathways were enhanced by strengthening the processes, policies, and documentation that surrounded organisational structure and human resources. Development of clear organisational structures facilitated communication between individuals in different departments and at different levels within the test facility. This was supported through development and implementation of key SOPs for regular, documented human resource support including appraisals and Curriculum Vitae review. Together, these had an additional effect on individuals’ sense of place and therefore, sense of value within the test facility. In some test facilities, new structures were put in place for requesting training for career development, and staff were adequately empowered to take up these opportunities. Across test facilities, but particularly in those that had achieved GLP certification, there were more job opportunities at the institution, with more studies an investment attracted to the test facility. (Quotes: INS1)
In-house training programmes were developed and delivered across test facilities including general training in GLP awareness, Quality Assurance, training in SOPs, Health and Safety/Fire training, archiving training, leadership training, and computer system validation and usage. Training programmes were often developed by test facility staff following attendance at externally delivered training courses. Implementation of training was overseen by staff in a range of roles, as a result of the additional responsibilities being taken on by staff at all levels. Test facility management noted that MSc and PhD students from institutions attached to their test facility had had the opportunity to train in a GLP environment as a result of the developed quality management systems. (Quotes: INS2)
Infrastructural improvements at test facilities enhanced the research environment including laboratory, office and shared spaces. Areas of test facilities that were built from scratch or were refurbished included: insecticide testing laboratories, molecular laboratories, insecticide spray rooms, bed net washing areas, insectaries and animal houses. Enhancements included installation of new equipment, improved separation between resistant and non-resistant mosquito strains in insectaries, construction of new facilities to allow new test types (for example, net washing facilities to allow testing of insecticide-treated nets), increased space within existing laboratories, and enhancements to working conditions (e.g. new benching, stools, and wipe-clean tiled surfaces). Installation of new equipment, such as PCR machines, facilitated establishment of new assays and meant that testing of samples could be conducted in-house, reducing the time to obtaining results. Non-laboratory facilities built or refurbished included office spaces, communal break and training areas, facility archives and computer server rooms. For both laboratory and non-laboratory facilities, this enhanced the working environment linked to individuals’ motivation, job satisfaction and pride in their jobs. (Quotes: INS4a)
The research environment was also strengthened through improvements in the procurement processes in some test facilities, and to IT infrastructure across all test facilities. Streamlined procurement processes included the implementation of quality management system practices initiated by the GLP project, in particular in the widespread use of SOPs. This simplified processes and made transfer of work responsibilities when colleagues were absent more seamless. IT infrastructure improvements were relevant across GLP and non-GLP studies, improving processes for accessing and storing study data, managing results in preparation for scientific reports and publications, and improving communication between staff within the test facility through, for example, more widespread use of email and installation of internal telephone systems. (Quotes: INS4b)
Whilst the project was focused at the institution level, secondary effects were identified at the individual level. These effects included extensive training, strengthening of career prospects, furtherment of careers, structured working practices and enhanced work motivation.
While areas covered by training programmes varied between test facilities, there was a substantial increase in both breadth and depth in all training programmes. Training examples cited included 24 topics or areas, encompassing training related to QMSs, science specific training, training relating to safety, and business, leadership and life skills training. The training programmes reached staff at all levels of the facility, including non-technical staff such as administrators, drivers, office attendants and gardeners. Training was often specifically tailored to the needs of the test facility staff. (Quotes: IND1)
This training, combined with the practical experience of working in a GLP-compliant laboratory, was highly valued in enhancing career prospects. In all test facilities, staff took on additional responsibilities through, for example, leading on fire safety and organising fire drills or chairing training committees.
Individuals felt an enhanced sense of professionalism and prestige associated with developing and working in a GLP-compliant test facility and seeing the effect of work they had been involved with on changes in vector control policies and practices. This enhanced motivation amongst test facility staff at all levels and technicians and non-scientific staff in particular felt that their work was more structured, meaningful and purposeful following the project (Quotes: IND4). This motivation was enhanced further by an improved working environment following infrastructure improvements, including more working space, air conditioning, and better-quality workstations. (Quotes: IND2)
Examples of career progressions and internal promotions within test facilities were cited across several locations, including promotion of laboratory technicians to laboratory supervisors, and laboratory supervisors to senior management positions. (Quotes: IND3)
At the national and international level, identified secondary effects included sharing of best practices within consortia and linked institutions, and the development of regional expertise related to data management and quality assurance.
Test facilities saw increased support from national level institutions, including increased investment in infrastructure. Alongside this, test facilities’ expertise in GLP was recognised at a national level, with the expectation that they would now act as national centres of excellence, both as a model of best practice and as a provider of training in entomology and relevant SOPs. Increased engagement with research outputs at the national decision-making level was anticipated as the next stage of this enhanced relationship with national level institutions, alongside a belief that this would raise policy-makers’ expectations of the test facilities’ performance. (Quotes: NAT1 and NAT2)
At a national and international level, the opportunity to meet and share experiences with the seven collaborating test facilities allowed best practice to be shared throughout the network, although this was not always fully realised as test facilities sought to strike a balance between collaboration and retaining a competitive advantage as a provider of product testing services. For construction and renovation of infrastructure, best practice was shared between test facilities that were geographically close together, because the requirements for buildings were the same and because travelling to these test facilities to see the buildings in person was easier. Data management and quality assurance expertise that was developed in test facilities further along the path to GLP certification, and by individuals associated with these test facilities, was also disseminated through the network. This was done formally through the project network, via training workshops and shared resources such as SOPs, and informally as these individuals acted in consultancy roles both within and outside of the institutions collaborating in the programme. Involvement in this network also raised the profile of individual test facilities, allowing these facilities to attract new studies and collaborators – including both GLP and non-GLP studies. (Quotes: NAT3)
Ripple effects of the project beyond research capacity strengthening were widely reported for both individuals and the community surrounding the institutions. At the individual level, these were particularly focused on the transfer of skills developed through training and new practices associated with GLP to home lives. This was particularly true in test facilities that had broad and inclusive training programmes, encompassing topics such as how the test facility was budgeting for GLP and including staff in roles across the test facility such as drivers/cleaners. Here, individuals noted how they had applied time management, organisation, and budgeting skills developed through the GLP project to managing their personal lives and households. (Quotes: IND4).
Effects on communities, which could be described as national level effects, were rooted in often locally sourced solutions to challenges and, in particular, procurement and infrastructure development. By being locally based and finding local solutions, communities around the test facility saw investment in local businesses for consumables, construction materials and construction teams. Also reported was an increase in local employment as new studies were attracted, creating roles such as mosquito collection for experimental hut studies, and improvements in shared infrastructure such as roads. Test facility staff who recognised these effects in the community both took pride in these effects and valued them highly. (Quotes: NAT4).
Despite a focus on the institutional level, the GLP laboratory capacity strengthening project had effects at each level of the research system – individual, institutional and national/international. These effects are summarised in Figure 1. Primary effects at the institutional level were the development of the GLP quality management system, the central goal of the project, which was achieved through improvements in the infrastructure, research areas and research environment, and including non-research departments such as procurement. This was complimented by enhanced internally delivered training programmes, documentation, human resources processes and organisational structures. Secondary effects at the individual level centred around training, career enhancement, resulting in increased motivation and job satisfaction, for individuals with diverse roles within the test facility. At the national/international level, the secondary effects of the GLP project were increased support and engagement from national level institutions, and the development of opportunities for inter-facility networks and sharing of best practice.
These findings align with factors previously identified for evaluation of research capacity strengthening initiatives22. The findings from this study emphasise that the “research team” included in evaluations of research capacity strengthening should include auxiliary, administrative and technical staff. Therefore, it is imperative that quality training is extended to these roles also, as happened in several test facilities within the GLP project, and that recognition of research leadership/esteem should also encompass recognition of excellence in these roles.
The programme was institutionally focused, with the end goal of achieving GLP certification. This, however, required inputs and investment at the individual level (especially training of key individuals, through external workshops or courses, who then went on to implement training in-house or across the network), at the national/international level (for example, by bringing test facilities together to facilitate international networks and collaboration), as well as at the institutional level (an extensive programme of construction and rehabilitation, development of documentation and training programmes, recruitment, and updated organisational structure). A direct effect at these levels was experienced because of this investment, but it also triggered effects across the boundaries between these levels, demonstrating that the three levels within research systems are interconnected (Figure 2).
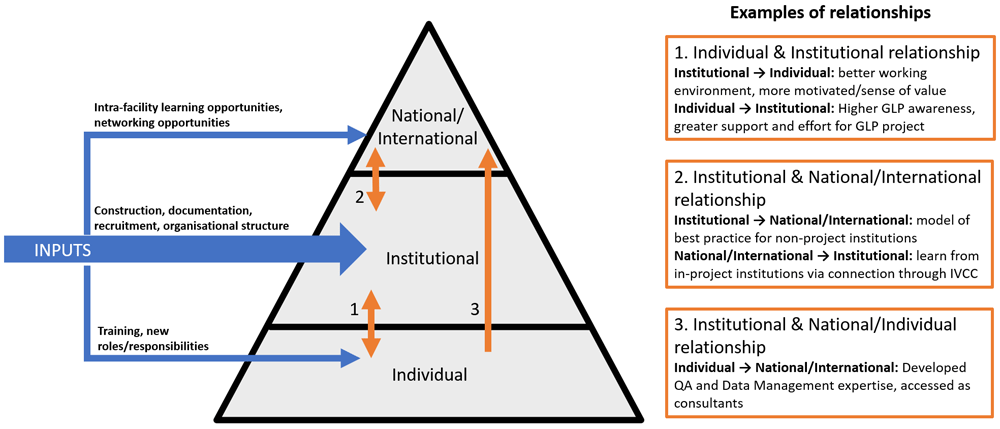
This finding supports calls for research capacity strengthening efforts to be explicitly aware of what is happening at all levels and to optimise this effect, even if the described goal is at a single level, in order to plan to optimise these ripple effects22–24. This may be particularly true for research capacity strengthening initiatives that are targeted at the institutional level, as there is scope for triggering effects across the boundaries with both individual and national/international level, and towards the institution. This also has implications for evaluations of research capacity strengthening initiatives that describe a goal at a single level. In this case, the effects triggered across the boundaries away from the institutional level and jumping directly from the individual to the national/institutional level are effects that contribute to a more broadly strengthened research system without being related to the single-level goal. Nevertheless, these effects are important to capture, both to accurately describe the totality of effects of a programme, but also because the ripple effect at the national/international and individual levels then has an effect of further strengthening at the institutional level.
Ripple effects were identified beyond the research system, with rich descriptions of how the GLP project was making a wider difference to the lives of the people and communities that surround the test facility (Figure 3). That these effects were meaningful to those engaged in the GLP project suggests that further exploration of these effects is warranted, and evaluations of similar programmes should expressly plan to capture information about these effects. This is because the ripple effects are an additional source of evidence to engage and motivate individuals in research capacity strengthening projects which, by their nature, have the potential to be challenging and burdensome during implementation.
Together, these findings show that the GLP project acted at and had primary and secondary effects at all three levels of the research system, that the relationship between these levels is complex and interrelated, and that there are ripple effects beyond the research system itself. These findings should, therefore, inform the design and evaluation of similar programmes to:
1. Use the three levels - institutional, individual and national/international - as the foundation for programme development, to promote a holistic approach to programme design, and inform evaluation of effect at each level22,23;
2. Explicitly plan for and capture information from each level about the interactions with other levels, and capture ripple effects22.
Many indicators for evaluating the outcomes and effect of research capacity strengthening initiatives at all three levels already exist, and these may form the basis of evaluations of similar projects7. Box 1 summarises some suggested areas for consideration when developing evaluations of institutional capacity strengthening projects. For ripple effects in particular a mixed methods or qualitative approach may be beneficial25,26.
Individual level
Broad definition of research team to include auxiliaries, technical staff and administrators, and outcome indicators for training of staff in these roles
Broad definition of recognition of leadership to include recognition of proficiency working in a high-quality research system
Consider the ripple effect of individual development of transferable life skills
Institutional level
Interrogate the uptake of training programmes to support career development, and the extent to which staff access these programmes.
Consider equity of access to these programmes (e.g. gender, role within institution)
Consider the extent to which training is integrated into the host institution, with a view to sustainable delivery
Consider unintended transferred learning from the research capacity strengthening project to non-research practices across the institution (e.g. to research management support systems) or other research areas
Consider the relationship between an improved research environment and staff motivation/job satisfaction
The strengths of this study are in the diversity of participants involved, capturing the views of staff filling a wide range of roles in five test facilities across three African countries. This approach ensured that effects meaningful to staff in diverse roles were reflected in the findings and offered a voice to staff less often heard within research teams, such as those of technicians and administrators. Furthermore, by using a qualitative approach, this study was able to richly describe the perceived effects of the GLP project and reveal and explain interactions between these effects.
This study is, however, limited by several factors. With a grounding in a specific laboratory capacity strengthening project, caution should be exercised on generalising these findings to all research capacity strengthening projects. Test facilities were at different stages towards GLP certification, with two test facilities having been granted GLP certification to date and this study is unlikely, therefore, to have captured all of the effects of the GLP project. Further effects will likely be identified by staff as the test facilities progress through certification and begin to attract GLP studies from multinational company sponsors. In addition, given the relatively small amount of time specifically dedicated to this question within the interviews, it is likely that additional effects may have been identified given more interview time. Finally, changes had to be made to data collection methods due to the COVID-19 pandemic: the responses at the two test facilities that participated via email and video-call are likely to be more superficial due to reduced opportunities to ask follow-up questions on observations.
Building research capacity in public health and related fields is essential to the generation of high quality, reliable scientific data. This study, focussing on a project supporting seven test facilities in Africa towards GLP certification, shows that research capacity strengthening interventions for laboratories with a focus on institutional level goals require actions also at individual and national/international levels. The effects of engagement at all three levels towards research capacity strengthening can be amplified by incorporating additional actions at the national/international level, particularly when many institutions are engaged in the same project. This does, however, require that each institution buys into the opportunities for inter-facility learnings for this to collaborative approach to work optimally. Furthermore, there are interactions that happen in both directions across the boundaries between the individual, institutional, and national/international levels, with effects at one level triggering a further effect at another level. These interactions can amplify the effects of an intervention, including research capacity strengthening effects which are the primary objective of such projects. Finally, there are additional “ripple effects” that extend beyond the research system, but that are meaningful to individuals engaged in these projects. The significance of these findings are twofold: firstly, it confirms the interactions between the levels of the research system and, therefore, adds to the evidence that research capacity strengthening projects should plan both to address and to evaluate their effects at all three levels; and secondly, it shows that it is possible to capture the ripple effects of investment in research capacity strengthening and that capturing these effects should be planned for explicitly at the instigation of the project to support further engagement of stakeholders in research capacity strengthening.
Transcriptions of interviews with facility staff are available from the research group on request (please email ccr@lstmed.ac.uk to request access), on a case by case basis for the purpose of informing further research and on the condition that it will not be published in part or in entirety. They have not been made available as a dataset because they cannot be de-identified without compromising anonymity and the ethical approval conditions for the project stated that only the research team would have access to the data.
Harvard Dataverse: Interview Guide and Information Sheets for: Developing laboratory capacity for Good Laboratory Practice certification: lessons from a Tanzanian insecticide testing facility. https://doi.org/10.7910/DVN/NADZPS18.
This project contains the following extended data:
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We thank the Bill & Melinda Gates Foundation, USAID and UK Aid for their support of the test facilities. We also thank Jameel Bharmal, for his support in data collection and translation, and Russell Dacombe, for his technical input.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am an arbovirologist working for CDC in global health in Kenya
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Laboratory and field oriented vector control research. I am also into use of scientific data to make sound public health decisions. I am also involved in capacity strengthening of institutions involved in public health entomology research in Nigeria.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Käser M, Maure C, Halpaap BM, Vahedi M, et al.: Research Capacity Strengthening in Low and Middle Income Countries - An Evaluation of the WHO/TDR Career Development Fellowship Programme.PLoS Negl Trop Dis. 10 (5): e0004631 PubMed Abstract | Publisher Full TextCompeting Interests: Although the reviewer himself does not have any co-authorships with any of the authors, the institution he works in has collaborations with two of the authors’ institutions. This fact, however, does not influence any of the reviewer’s comments.
Reviewer Expertise: Basic research, cell biology, immunology, molecular genetics Technology development and application, with focus on neglected tropical diseases (NTDs) Capacity building with focus on NTDs and in LMICs Evaluation of research capacity strengthening programmes
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 10 May 21 |
read | read | ||
|
Version 1 27 Nov 20 |
read | read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)