Keywords
Pneumococcal conjugate vaccine, vaccine evaluation, vaccine coverage, vaccine uptake, Brazil, Colombia, Peru, income level, childhood pneumonia mortality
Pneumococcal conjugate vaccine, vaccine evaluation, vaccine coverage, vaccine uptake, Brazil, Colombia, Peru, income level, childhood pneumonia mortality
Vaccination programs targeting pneumococcus are now established globally. Pneumococcal conjugate vaccines (PCVs) have effectively reduced the burden of disease and death due to pneumonia and other severe pneumococcal infections (e.g., septicemia, meningitis)1–7. For instance, national-level analyses found that estimated declines in pneumonia mortality among children 2–11 months of age were 8% (95% credible interval (CrI): 2 to 14%) in Brazil, 14% (-5 to 35%) in Colombia, and 36% (17 to 44%) in Peru7. The impact of PCVs on preventing deaths due to pneumonia could differ between higher- and lower-income populations. Differences in the etiology of pneumonia, the distribution of pneumococcal serotypes, and access to healthcare can influence the proportion of deaths that are preventable. Variations in vaccine uptake can also influence the overall reduction.
The countries in the Latin America and Caribbean region are ideal for conducting evaluations of the impact of PCVs. Countries in this region were among the first lower- and middle-income countries to introduce PCVs. The strong health and mortality data systems in these countries make it possible to perform detailed vaccine evaluation studies at a subnational level and to evaluate how variations in vaccine uptake relate to changes in rates of pneumonia deaths. The countries also have a wide variation in socioeconomic status at a subnational level, which provides a unique opportunity to compare the impact of PCV by income level. Therefore, this study aims to evaluate how the association between increasing vaccine uptake and population-level declines in death due to pneumonia varies by socioeconomic status in Brazil, Colombia, and Peru.
In a previous study, we evaluated the population-level impact of PCVs against pneumonia mortality at the national level in ten Latin American and Caribbean countries7. Among the 10 countries included in the national-level study, Brazil, Colombia, and Peru had subnational data on mortality, socioeconomic status, and vaccine coverage with a high spatial resolution, which enabled us to conduct this analysis. Mortality data were obtained from the National Mortality Information Systems. Both primary and non-primary causes of deaths were recorded for Brazil and Peru, while only primary causes were available for Colombia. The cause of death was classified using the International Statistical Classification of Diseases and Related Health Problems tenth revision (ICD-10) codes. Each country conducted standardized data cleaning and quality control8. We extracted mortality data for children at the municipality level that were available during the study period (2005-2015). The quality of mortality data was assessed and reported in the previous study7. Durations of pre- and post-PCV periods for each country are described in Table 1.
| Brazil | Colombia | Peru | |
|---|---|---|---|
| Pre-PCV period | January 2005 - February 2010 (5 years and 2 months) | January 2005 - October 2011 (6 years and 10 months) | January 2005 - July 2009 (4 years and 7 months) |
| Post-PCV period | March 2010 - December 2015 (5 years and 10 months) | November 2011 - December 2015 (4 years and 2 months) | August 2009 - December 2014 (5 years and 5 months) |
| Total number of J12-18 among children 2–23 months of age in the study period (coded as primary or non-primary causes of death)1 | 16,303 | 4700 (primary only) | 6854 |
| Total number of J12-18 among children 2–23 months of age in the study period (coded as primary cause of death)1 | 35,259 | 4700 | 5818 |
| Weighted average of PCV third dose coverage in the last year of the study period | 91.7% in 2015 | 91.1% in 2015 | 81.8% in 2014 |
An outcome of our analysis was all-cause pneumonia deaths, defined as having an ICD-10 code in the range of J12-J18. For Brazil and Peru, we used J12-J18 recorded as either primary or non-primary causes of death as the outcome in the main analysis, and used J12-J18 recorded as the primary cause of deaths as the outcome in the sensitivity analysis. For Colombia, we used J12-J18 recorded as the primary cause of death as the outcome, as they did not have data on non-primary causes of death.
We analyzed data for children 2–23 months of age, as our previous national-level analysis did not detect any changes in pneumonia mortality among older children (24–59 months of age) following the introduction of PCV in any of the ten countries we analyzed7. Children under two months of age were not included in the analysis for both biological and practical reasons7.
For Brazil and Colombia, we classified municipalities into three income levels (low, medium, and high) using the Gross Domestic Product (GDP) per capita for the year of PCV introduction. For Peru, these three categories of the income level were created based on a five-level income level indicator, reflecting the per capita income in the year of PCV introduction. This indicator is obtained from household surveys and is based on quintiles of household per capita expenditure as it relates to the costs of a “basic consumption basket.”9,10 These income categories were defined for each country separately and cannot be directly compared across countries. We then created subnational regions by grouping municipalities based on these three income-level categories in each of the departments (n=32 in Colombia and n=25 in Peru) or states (n=27 in Brazil). For example, Peru had 75 possible combinations of departments and income levels; however, there were only 68 subnational regions simply because some strata did not exist (e.g., no low-income regions in Lima). Furthermore, we excluded subnational regions from the analysis if there were less than ten all-cause pneumonia mortality per year on average. After this exclusion, we had 49 subnational regions in Brazil (11 low-, 25 medium- and 13 high-income regions), 16 in Colombia (12 medium- and four high-income regions), and 23 in Peru (five low-, 10 medium-, and eight high-income regions) in the final analysis.
Brazil introduced the 10-valent PCV with a 3+1 schedule (2, 4, and 6 months + 12–18 months of age) in March 2010. Colombia introduced the 10-valent PCV in November 2011 and used a 2+1 schedule (2 and 4 months + 12 months of age). Peru introduced the 7-valent PCV with the 2+1 schedule in August 2009 (2 and 4 months + 12 months of age), and switched to the 10-valent PCV with the 2+1 schedule in December 2011.
Data on the PCV third dose coverage (i.e., last primary dose for Brazil and booster dose for Colombia and Peru) by year were obtained from the National Immunization Programs at the municipality level in all three countries. For Colombia and Peru, we first conducted linear interpolation for missing coverage data, if there were any, in each municipality. We then calculated a weighted average of the coverage in each subnational region. We used the population size of each municipality as a weight for Brazil and Colombia. For Peru, a total number of deaths during the study period in each municipality was used as a weight, as municipality-level population data were not available.
To estimate changes in pneumonia mortality among children after the introduction of PCVs, we fit the following Poisson regression model to the data from each country separately such that
The outcome, Yi,t, is the number of all-cause pneumonia deaths in subnational region i in year t. To control for unmeasured temporal factors affecting pneumonia mortality, we included all-cause deaths other than those caused by respiratory illness as a covariate in the model (xi,t) where β1i represents the region-specific regression parameter that describes the association between this covariate and deaths and β0i is the region-specific intercept. To account for potential overdispersion and unexplained correlation in deaths across time, we included an observation-level random effect, ϕi,t, that follows a first order autoregressive process. The weighted average of the PCV third dose coverage in subnational region i in year t is given as vi,t and the region-specific vaccine effect, θi, is modeled as a function of region-level socioeconomic status such that
where , , and are indicator variables for the income level. The remaining variation in θi that is not explained by the effect of income level is captured by ηi which are modeled using independent Gaussian distributions with zero mean and shared variance parameter.
Posterior medians were used as point estimates and the 95% highest density CrIs were calculated to quantify uncertainty. All analyses were performed in R (Vienna, Austria)11. The aggregated time series data and code can be found in the following GitHub repository: https://github.com/weinbergerlab/PAHO_subnational. A stable copy of the repository is available from https://zenodo.org/badge/latestdoi/291341482. More details on the model, such as the information on prior distributions, can be found in the Extended data, Supplementary methods12.
We evaluated how the population-level impact of PCV changes by income level in a few different ways. First, we quantified declines in all-cause pneumonia deaths based on the actual coverage in the last year of the study period in each subnational region. Next, to compare the effect of PCVs between regions while holding vaccine uptake constant, we estimated declines in all-cause pneumonia deaths that would be expected with 90% uptake of the third dose of PCV. More details on the calculations are described in the Extended data, Supplementary methods12.
The uptake of PCV increased rapidly within a few years after introduction in all countries, but there was variation by region (Extended data, Figure S1)13. For example, although the average uptake of the third dose was 82% at the national level in the last year of the study period in Peru (Table 1), average uptake varied from 55% to 100% between regions. Uptake of the third dose was higher in more affluent regions (Extended data, Figure S2)13. For example, in Peru, the median uptake was 67%, 88%, and 90% in low, medium, and high-income regions, respectively, in 2014.
All-cause pneumonia mortality declined over time across income levels and countries, and these declines started before the introduction of PCV (Figure 1A). This trend became unclear after adjusting for all-cause mortality other than those caused by respiratory illness (Figure 1B). In the last year of the study period, most subnational regions across countries observed declines in all-cause pneumonia deaths with large uncertainties, while low-income regions in Peru experienced larger declines (Figure 2). Higher uptake of the third dose of PCV was associated with additional declines in pneumonia deaths in most of the regions in all three countries (Figure 3). In Peru, this association was especially strong in low-income regions, while it was modest among high-income regions and not clear among medium-income regions.
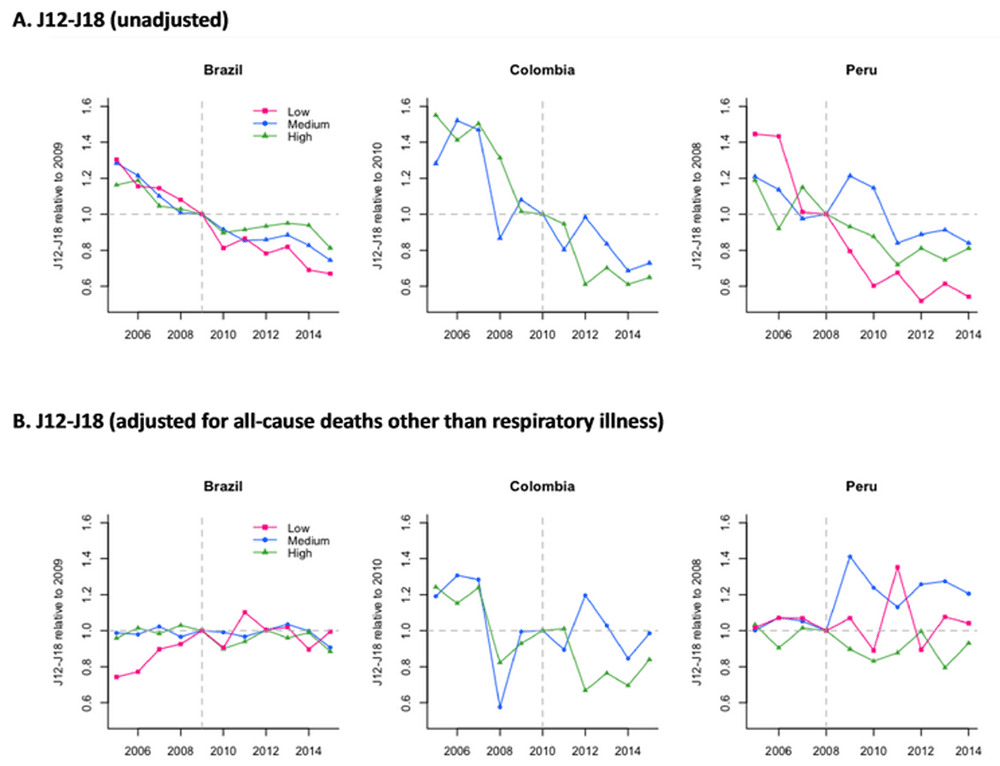
Pink, blue, and green lines represent estimates for low-, medium-, and high-income subnational regions in each country. Vertical dashed lines represent the final year in the pre-PCV period in each country.
Abbreviations: ICD-10, International Statistical Classification of Diseases and Related Health Problems tenth revision; PCV, pneumococcal conjugate vaccine.

Pink, blue, and green bubbles represent the point estimates for low-, medium-, and high-income subnational regions in each country. Grey bars are the 95% CrIs. The size of bubbles is proportional to the number of all-cause pneumonia deaths in the last year of the pre-PCV period. Horizontal dashed line indicates one, meaning that there were no changes in all-cause pneumonia mortality. In panels on the right, diamonds represent the average declines in each income level.
Abbreviations: PCV, pneumococcal conjugate vaccine; CrI, credible interval.
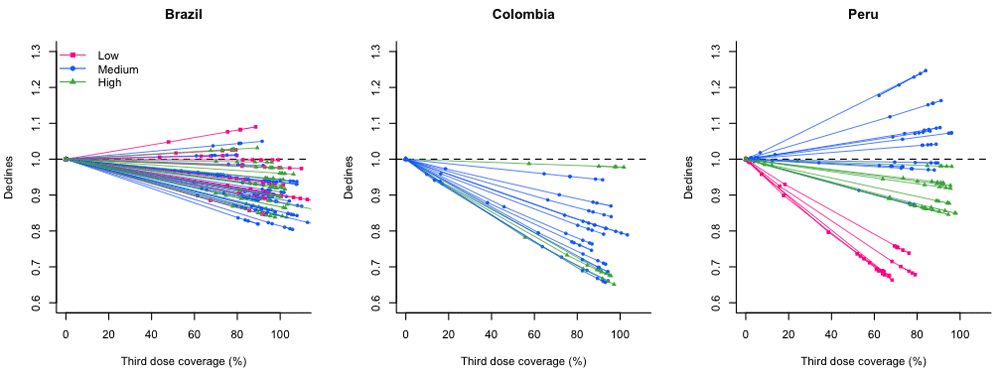
Pink, blue, and green dots represent estimates for low-, medium-, and high-income subnational regions in each country. Horizontal dashed line indicates one, meaning that there were no changes in all-cause pneumonia mortality.
Abbreviation: PCV, pneumococcal conjugate vaccine.
With 90% uptake of the vaccine, we estimated declines in all-cause pneumonia would range from 4–38% in low-income regions, -4–23% in medium-income regions, and 8–25% in high-income regions (Table 2). The estimates for individual regions had a high degree of uncertainty due to small numbers of deaths (Figure 2). There was no evidence that the effect of the vaccine on pneumonia deaths differed between high- and low-income regions in Brazil and Colombia. In Peru, estimated declines in pneumonia mortality were largest among low-income regions. The estimated decline in pneumonia mortality in low-income regions was 52% (95% CrI: 14%, 90%) and 38% (-2%, 76%) smaller than that in medium-income regions and high-income regions, respectively.
For Brazil and Peru, we repeated the analysis but only included deaths where pneumonia was recorded as the primary cause of death as the outcome variable. This modification did not appreciably change the estimated declines in Brazil, while it increased the estimated impact of PCV in Peru (Extended data, Table S1)12. The discrepancy in Peru was due to changes over time in the proportion of pneumonia deaths recorded as the primary cause vs contributing cause of death (Extended data, Figure S3)13, and this shift exaggerated the estimated impact of PCV when analyzing the primary cause of death only.
Using spatially disaggregated data, we evaluated whether the population-level effect of PCV among children 2–23 months of age varies between income levels in Brazil, Colombia, and Peru. High-income regions had higher coverage of the third dose of PCV. The estimated decline in all-cause pneumonia deaths associated with increasing PCV uptake was similar across income levels in Brazil and Colombia, while there was a larger decline among low-income regions in Peru.
To date, seven post-licensure studies have evaluated the impact of PCV on pneumonia mortality, all of which were conducted in Latin American and Caribbean regions1–7. These studies used various datasets and methods, and the magnitude of reported effects varied. For example, Diaz et al. conducted a nested case-control study in Chile and estimated that pneumonia deaths declined by 71.5% (95% CI: 9.0-91.8%)2. On the other hand, a multi-country study conducted time series analysis and did not find declines in Guyana and Honduras due to large uncertainty7.
A previous study reported larger PCV-associated declines in pneumonia mortality among children in low-income municipalities in Brazil6, while we did not see differences across income levels. That study used three indicators (human development index, children in poverty, and maternal primary education), while we used GDP per capita in the year of PCV introduction. Among children 2–23 months of age, the previous study found the largest decline in low-income regions only when municipalities were grouped based on maternal primary education. Our income classification based on GDP is most closely related to their HDI-based classification. With this HDI category, the previous study found that medium-level regions observed slightly larger declines than low- and high-level regions, which is consistent with our findings for Brazil (Figure 2 and Table 2).
Data sparsity often becomes an issue when working with subnational data. As death is a rare clinical outcome compared to others (e.g., hospitalizations, outpatient visits), both our outcome (J12–18) and controls (other ICD-10 chapters) had small counts per unit time at subnational level. To reduce the noise introduced by sparse counts, we aggregated data by year. When aggregating time series data by a larger time interval, models will be fit to fewer data points, which may also cause an issue, such as increased uncertainty in estimates.
Our study has some limitations. The quality of mortality data varied by country and changed over time, as discussed in detail in the national-level study7. To minimize the influence of data quality, each country collected and cleaned the data using standardized methodology in collaboration with the Pan American Health Organization (PAHO). We also controlled for changes in data quality by including the control cause of death in the model, assuming that changes in data quality affect both the outcome and the control. Our results, however, should be interpreted carefully. Regarding the data on PCV uptake, some municipalities in Brazil had unreliable data exceeding 100% with abrupt increases and decreases. We defined the income level of each municipality using the data in the year of PCV introduction, although the income level could change over time. For trend adjustment, we were only able to include one control cause of death (all-cause deaths other than those caused by respiratory conditions) due to data sparsity. In Peru, even common control causes of death only had 0–2 deaths per year in many regions. However, we believe that all-cause deaths were able to successfully adjust for some key trends because its estimated coefficient was different from zero in many regions.
One challenge with the type of analysis conducted here is that vaccine uptake data tend to be less reliable at local scales. Estimating both the numerator (number of children vaccinated) and denominator (number of children eligible for the vaccine) becomes more challenging at small spatial scales. Because the vaccine uptake data were measured with error, the estimates for the association with pneumonia deaths could be biased towards zero. This trend was clear especially among small low-income regions in Peru which were filtered out from the final analysis. Having higher quality data on vaccine uptake at a local level could facilitate robust estimates of vaccine impact in other countries and regions.
In conclusion, in three middle-income countries in South America, regions with higher PCV coverage experienced larger declines in pneumonia deaths, regardless of the income level. Focusing public health efforts on increasing the vaccine coverage could prevent additional childhood deaths.
Zenodo: AHO_subnational 0.2; http://doi.org/10.5281/zenodo.401109714.
Subfolder ‘Data’ contains the aggregated time series data used in this study. Data are also available at https://github.com/weinbergerlab/PAHO_subnational/tree/master/Data.
br.meso.v1.csv, co.meso.v1.csv, and pr.dept.inc.v1.csv include time series for subnational-level mortality data for Brazil, Colombia, and Peru, respectively. pr.cov.by.dept.inc.csv includes the PCV coverage data for Peru.
Figshare: OnlineSupplement.pdf; https://doi.org/10.6084/m9.figshare.12934901.v112.
This file contains the following extended data:
Supplementary methods
Table S1. Average declines in all-cause pneumonia mortality among children aged 2–23 months expected with the 90% coverage of PCV by income level in Brazil and Peru using J12-18 coded as the primary cause of death as an outcome.
Figshare: Supplementary Figures; https://doi.org/10.6084/m9.figshare.12957887.v113.
This file contains the following extended data:
Figure S1. Uptake of the third dose of pneumococcal conjugate vaccines by subnational region in Brazil, Colombia, and Peru.
Figure S2. Coverage of PCV third dose in the last year of the study period by income level in Brazil, Colombia, and Peru.
Figure S3. Proportion of J12-J18 recorded as the primary cause of deaths among J12-J18 recorded as any causes of deaths in Brazil and Peru.
Analysis code available from: https://github.com/weinbergerlab/PAHO_subnational.
Archived analysis code at time of publication: http://doi.org/10.5281/zenodo.401109714.
All data and code are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank Carlos Andrés Castañeda-Orjuela and Diana Patricia Diaz Jimenez from the National Institutes of Health, Colombia; and Juan Pablo Alzate Granados from the Fundación Universitaria de ciencias de la Salud, Colombia; for support in data collection and review.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious disease epidemiology; vaccinology, paediatrics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbial Pathogenesis; Mucosal Immunity; Vaccine Preventable Diseases
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 22 Sep 20 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)