Keywords
Postpartum family planning, cost savings, cost effectiveness analysis, LARC, faith-based, pregnancy averted, access, contraceptive
This article is included in the International Conference on Family Planning gateway.
Postpartum family planning, cost savings, cost effectiveness analysis, LARC, faith-based, pregnancy averted, access, contraceptive
A multitude of variables influence maternal and child health outcomes, such as delivery of and access to antenatal care, giving birth in facilities, and many more. However, the postpartum period, is equally important and often overlooked. The evidence of reduced maternal mortality upon engagement in postpartum family planning (PPFP), and the absence of robust PPFP programs globally, leads the World Health Organization (WHO) to distinguish the postpartum period as a key opportunity for promoting the health of mothers and babies1.
Women spend on average about 30 years, or three-quarters of their reproductive lives, attempting to avoid pregnancy2. Globally, there is a large unmet need for family planning in the postpartum period: 90% of women in this group want family planning, for birth spacing or to avoid unintended pregnancies and stop child bearing once desired family size has been reached. In Rwanda, where there has been a coordinated response to maternal and child health; family planning provides a platform to continue the nation’s trend of improved maternal and child health. The WHO recommends at least 24 months between a birth and the next pregnancy for improved maternal and child health outcomes3. In total 76% of Rwandan women want PPFP and a 26% unmet need remains4–6. Despite this, about one half of births are conceived before the recommended interval of 24 months7.
Faith-based health facilities make up 30% of Rwanda’s healthcare system and fill critical gaps in care8,9. Some denominations do not include comprehensive contraceptive options, leading to possible barriers to access10,11. “More effective” family planning methods, such as those included in this analysis, remain absent at these facilities10. Thus, attention to potential cost savings with the inclusion of these methods at all of Rwanda’s health facilities warrants further attention.
This cost effectiveness analysis (CEA) compared two categories of family planning methods of postpartum women of reproductive age (12–49 years) in Rwanda. Each consisted of one of the two most utilized methods in Rwanda: longer-acting reversible contraceptives (LARC) identified as injections and subdermal implants; and shorter-acting reversible contraceptives (non-LARC), pills and condoms12. A total of 45% of women do not use contraception postpartum, and this comparator is also represented in the model13.
This CEA compared two interventions, addressing whether the additional cost of LARC is justified by the additional health benefits from a health systems perspective (Figure 1). TreeAge Pro 2018 R1 was used to develop the model and run the sensitivity analysesa. A time horizon of 24 months was used to reflect the WHO suggested two-year spacing from birth until the next pregnancy, with time zero designated as time of birth.
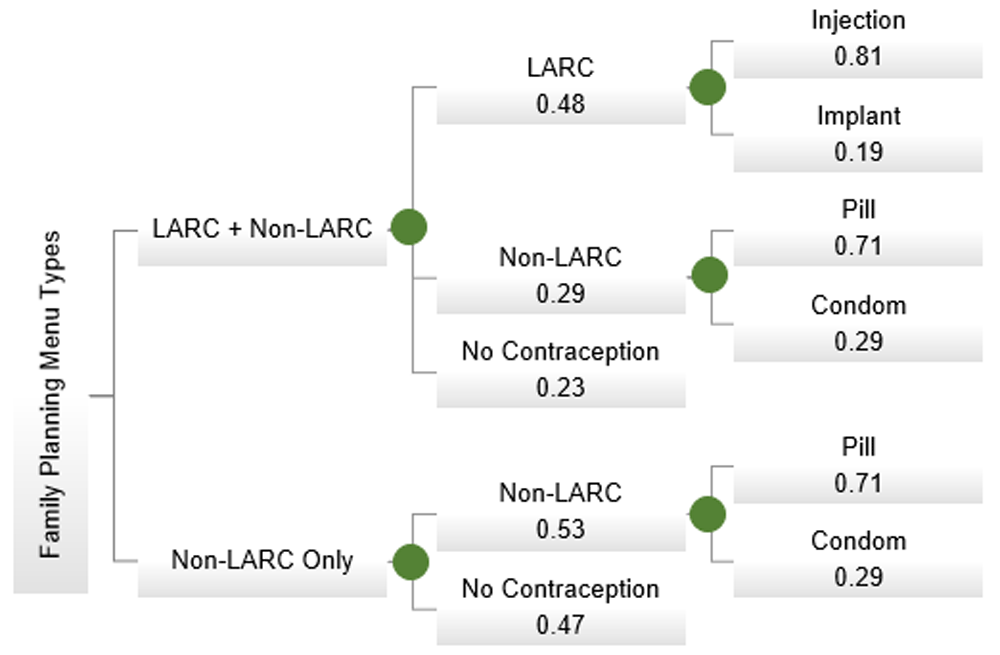
One includes the most common longer-acting reversible contraceptives (LARC), injections and subdermal implants; the second only provides the most common shorter-acting reversible contraceptives (non-LARC), pills and condoms12. Decision nodes are indicated with a circle; probability values are listed below each type and method.
Table 1 displays model input values collected from Rwanda-specific sources or other similar environments when Rwanda specific values were not available. Usage rates and discontinuation probabilities are specific to postpartum women in sub-Saharan Africa. Costs and chance of pregnancy were modeled for 24 months; single pregnancy incidence and costs were modeled for 12 months (two pregnancies postpartum is biologically unlikely for this timeframe). All cost data was: converted to dollars cost in Rwanda based on Gross Domestic Product (GDP) per capita, adjusted to 2018 US$ for the first year, and applied a 3% inflation rate for the second year of the 24-month time frame for the analysis. Key inputs from Table 1 were used to model base-case results in TreeAge Pro 2018.
| Cost Inputs | Estimate 2 years (USD$) | Probability | Source | Country/ Org |
|---|---|---|---|---|
| LARC | ||||
| Injectables | $22.15 | 0.509 0.807* | RDHS (2016)13 | Rwanda |
| Commodities | $11.70 | Singh et al. (2012)15 | UNFPA | |
| Supplies | $1.40 | Singh et al. (2012)15 | UNFPA | |
| Labor | $8.72 | Singh et al. (2012)15 | UNFPA | |
| discontinuation rate^ | 0.41 | RDHS (2016)13 | Rwanda | |
| chance of pregnancy | 0.0003 | RDHS (2016)13 | Rwanda | |
| Implant | $18.76 | 0.122 .193* | RDHS (2016)13 | Rwanda |
| Commodities | $16.28 | Singh et al. (2012)15 | UNFPA | |
| Supplies | $0.38 | Singh et al. (2012)15 | UNFPA | |
| Labor | $1.82 | Singh et al. (2012)15 | UNFPA | |
| discontinuation rate^^ | 0.03 | RDHS (2016)13 | Rwanda | |
| chance of pregnancy | 0.01 | RDHS (2016)13 | Rwanda | |
| Non-LARC | 0.527* | |||
| Male Condom | $10.05 | 0.056 0.288* | RDHS (2016)13 | Rwanda |
| Commodities | $5.80 | Singh et al. (2012)15 | UNFPA | |
| Supplies | $- | Singh et al. (2012)15 | UNFPA | |
| Labor | $4.11 | Singh et al. (2012)15 | UNFPA | |
| discontinuation rate* | 0.57 | RDHS (2016)13 | Rwanda | |
| chance of pregnancy | 0.3276 | RDHS (2016)13 | Rwanda | |
| after discontinuing injectable, using condom | 0.0945 | RDHS (2016)13 | Rwanda | |
| after discontinuing implant, using condom | 0.18 | RDHS (2016)13 | Rwanda | |
| Pills | $21.23 | 0.138 0.711* | RDHS (2016)13 | Rwanda |
| Commodities | $14.21 | Singh et al. (2012)15 | UNFPA | |
| Supplies | $- | Singh et al. (2012)15 | UNFPA | |
| Labor | $6.62 | Singh et al. (2012)15 | UNFPA | |
| discontinuation rate | 0.38 | RDHS (2016)13 | Rwanda | |
| chance of pregnancy | 0.172 | RDHS (2016)13 | Rwanda | |
| after discontinuing injectable, using pill | 0.0461 | RDHS (2016)13 | Rwanda | |
| after discontinuing implant, using pill | 0.09 | RDHS (2016)13 | Rwanda | |
| No Contraception | 0.175 0.474* | RDHS (2016)13 | Rwanda | |
| chance of pregnancy | 0.98 | RDHS (2016)13 | Rwanda | |
| after discontinuing injection** | 0.623 | RDHS (2016)13 | Rwanda | |
| after discontinuing implant | 0.86 | RDHS (2016)13 | Rwanda | |
| Discount rate+ | 3% | |||
| Pregnancy Costs | ||||
| Antenatal care++ | $28 | 1 | Hitimana et al. (2018)20 | Rwanda |
| Hospitalization | $3.72 | 1 | Vlassoff et al. (2015)18 | Rwanda |
| Normal vaginal delivery** | $17.48 | 0.93 | Rwanda MOH (2011)19 | Rwanda |
| Obstructed labor (C-section) | $43.66 | 0.0694 | Blaakman et al. (2008)17 | Rwanda |
*Weighted value for tree established based on probability with consideration for Rwanda LARC prevalent methods only
**Value established from range
^Modeled discontinuation after 1 year of use (1 year postpartum) with consideration for effectiveness tapering (effectiveness is maintained for 6 months following missed injection date); see limitation #3
^^Modeled discontinuation after 1 year of use (1 year postpartum)
+Applied to all cost inputs21
++Cost for antenatal care established assuming most women attend two visits: Visit 1 + (Visit 2 + Visit 3 + Visit 4)/3
The use of LARC methods saved $18.73 per pregnancy averted compared to contraceptive selections with non-LARC methods only (Table 2). When LARC is included in the menu, LARC is the dominating option among the contraceptive methods – the use of this contraceptive type both saves money and averts unwanted pregnancies with higher probability when compared with the non-LARC or no contraception use options.
Providing LARC postpartum family planning methods in the menu of options saves $18.73 per pregnancy averted compared to family planning options that offer non-LARC methods exclusively, for women of reproductive age (12–49 years) in Rwanda for two years following birth from a health systems perspective.
| Net Costs | Savings | Pregnancies | Pregnancies (Averted) | Cost Saved per Pregnancy Averted | |
|---|---|---|---|---|---|
| Non-LARC | $44.39 | N/A | 0.72 | N/A | N/A |
| LARC | $38.77 | $5.62 | 0.42 | 0.30 | $18.73 |
One-way sensitivity analyses were executed for variables with greatest impact to the model output. Input ranges were set to 50–150% of original value (Figures 2a–c).
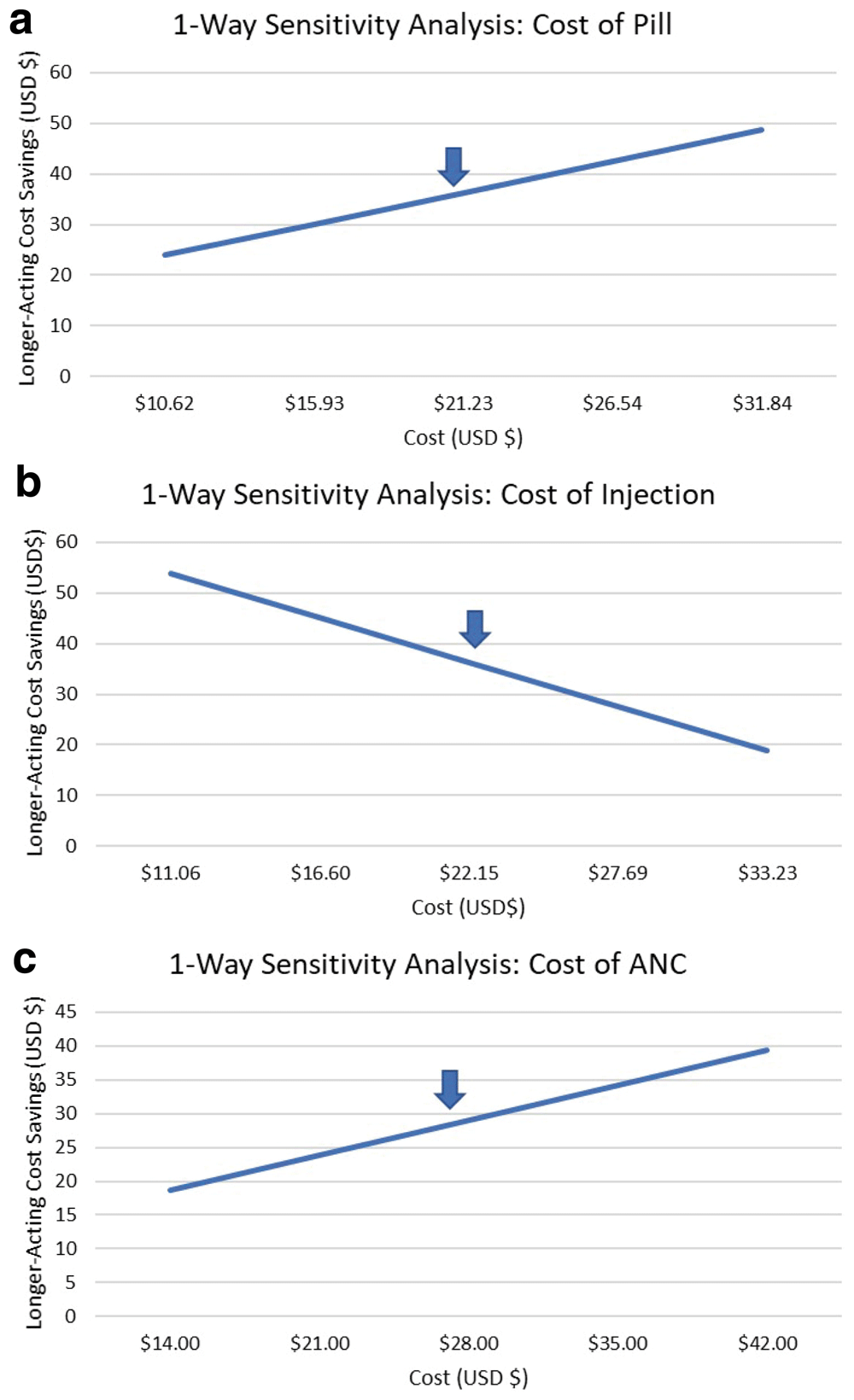
(a) When the cost of the pill (non-LARC method) is varied from $10.62–$31.84, savings due to the inclusion of LARC increases from $23.91–$48.75. (b) When the cost of an injectable (LARC method) is varied from $11.06–$33.23, savings due to the inclusion of LARC decreases from $53.80–$18.87. (c) When cost of antenatal care is varied from $14–$42, savings due to the inclusion of LARC increases from $18.68–$39.38.
There is uncertainty surrounding the following elements:
1. Study population – This analysis is limited to the evaluation of women in Rwanda who access care at a public facility. It fails to capture those who do not seek care at a government institution. However, since about 92% of Rwandan women deliver at a public healthcare facility, our analysis reflects the majority of the population13.
2. Contraception type – This analysis included two LARC methods and two non-LARC methods and therefore excludes some other contraceptive options. These four methods make up 77% of the contraceptive uptake in Rwanda and therefore is a reasonable representation of the population12. Yet, possible effects of the introduction of LARC methods on the methods not portrayed here could change the results. However, since these other methods are less effective than LARC, their inclusion is unlikely to diminish the estimated cost-effectiveness of LARC.
3. Tapering effectiveness upon discontinuation of injection method – It is reported that the injection method continues to provide protection for nine months following the last injection22. This phenomenon is modeled through an altered discontinuation rate if injection follow-up stopped at nine months postpartum.
This evaluation illustrates the inclusion of LARC methods in contraceptive options results in savings of $18.73 per pregnancy averted compared to family planning menus that exclusively include non-LARC methods for women in Rwanda for two years following birth. With Rwanda’s current population of 12.8 million, a birth rate of 32.23/1,000, and a 37% unplanned birth rate, $2.8 million US$ per year can be saved if LARC is included as a contraceptive choice across all health centers7,23,24. Cost savings provides the opportunity for the Rwanda Ministry of Health to apply this money to other high value interventions. With the incorporation of a greater than two-year postpartum window, additional cost savings is projected. This model does not capture the health benefits to the mother and second baby incurred if the two-year minimum window is observed. Future areas of research include the analysis of barriers and facilitators to PPFP uptake. With consideration for the country’s low postnatal care attendance rate, the integration of PPFP counseling into the facility discharge protocol is key for the benefits of LARC PPFP to be actualized.
All data underlying the results are available as part of the article and no additional source data are required.
This work was supported by the Bill and Melinda Gates Foundation [OPP1181398].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The preliminary stage of this work was supported by the University of California San Francisco Global Health Master’s Program. Carolyn Smith Hughes, MSc, provided guidance and support throughout this investigation. Dr. Felix Sayinzoga, Maternal, Child and Community Health Division, Rwanda Ministry of Health, Rwanda Biomedical Center, provided in-country support.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious disease epidemiology, HIV prevention and treatment, family planning, cost-effectiveness
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 27 Aug 19 |
read | read | |
|
Version 2 (revision) 03 Jun 19 |
read | ||
|
Version 1 18 Mar 19 |
read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)