Keywords
Complementary feeding, low- and middle-income countries, network meta-analysis, height-for-age, stunting, child development
Complementary feeding, low- and middle-income countries, network meta-analysis, height-for-age, stunting, child development
Linear growth is a marker of healthy childhood progression, closely linked with neurodevelopment in early life1. Despite global improvements in maternal, newborn, and child health (MNCH), the rate of children that fail to achieve their linear growth potential in low- and middle-income countries (LMICs) is high2. Prevention of linear growth faltering, also known as stunting (low height for age), during the complementary feeding period (6 to 24 months of age) is critical, since stunting during this life stage can have immediate, short- and long-term consequences that are difficult to reverse3–5. Continued malnutrition in children experiencing stunting can result in increased susceptibility and frequency to infections, as well as enhanced likelihood of cognitive, motor, and language impairment2,6. In later life, stunted children may also experience reduced life chances such as poor academic performance that may affect future earnings and increased risk for chronic diseases, including obesity if accompanied by excessive weight gain in adulthood5,7–9.
As children begin to wean off breastfeeding, there is a critical and continual need to ensure proper nutrition, hygiene, control of infectious diseases, and overall care during the complementary feeding period. Despite multiple factors playing a role in child’s linear growth, the majority of the reviews concerning linear growth for children during this life period in the past have focused on a single intervention domain (e.g. micronutrients) (Table 1). The comparative effectiveness of interventions is not clear across multiple domains, such as micronutrients, food supplements, deworming, maternal education, and water, sanitation, and hygiene (WASH) that could be important solutions to optimize linear growth during this life period. Additionally, all of the existing reviews have implemented a traditional pairwise meta-analysis that only allows for comparison between two interventions that have directly been compared head-to-head in clinical trials. Given that the majority of trials have used placebo or other comparators with limited clinical interest, the utility of pairwise meta-analysis can be limiting, particularly when assessing the broad sets of interventions that could be provided during the complementary feeding period.
| Study ID | Title | Interventions domains | No of studies | Types of studies included |
|---|---|---|---|---|
| Dangour 201322 | Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children (Review) | WASH | 14 | RCTs, cluster-RCTs, quasi- and non-randomised trials, controlled cohort or cross-sectional studies and historically controlled studies |
| Darlow 201623 | Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants (Review) | Micronutrient: Vitamin A | 10 | RCTs |
| Das 201324 | Micronutrient fortification of food and its impact on woman and child health: a systematic review | Micronutrients | 201 | RCTs, quasi-experimental and before-after studies. |
| De-Regil 201125 | Intermittent iron supplementation for improving nutrition and development in children under 12 years of age (Review) | Micronutrient: Iron (intermittent) | 33 | RCTs and quasi-RCTs with either individual or cluster randomisation |
| De-Regil 201326 | Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review) | Home fortification | 8 | RCTs or quasi-RCTs |
| Devakumar 201627 | Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: a systematic review and meta-analysis. | Micronutrient: MMS | 9 | RCTs, cluster-RCTs |
| Gaffey 201328 | Dietary management of childhood diarrhea in low- and middle-income countries: a systematic review. | Diet for diarrhea management | 29 | RCTs |
| Gough 201429 | The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials | Antibiotics | 10 | RCTs |
| Imdad 201130 | Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: a meta-analysis of studies for input to the lives saved tool | Micronutrient: Zinc | 36 | RCTs |
| Imdad 201731 | Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age (Review) | Micronutrient: Vitamin A | 45 | RCTs, Cluster-RCTs |
| Kristjansson 201532 | Food supplementation for improving the physical and psychosocial health of socio- economically disadvantaged children aged three months to five years | Food supplementation | 26 | RCTs and studies with historical controls |
| Lassi 201333 | Impact of complementary food and education on complementary food on growth and morbidity of children less than 2 years of age in developing countries: a systematic review | Complementary foods | 16 | RCTs, nonrandomized trials |
| Matsungo 201734 | Lipid-based nutrient supplements and linear growth in children under 2 years: a review | Lipid supplements | 7 | RCTs |
| Mayo-Wilson 201435 | Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age (Review) | Micronutrient: Zinc | 80 | RCTs |
| Pasricha 201336 | Effect of daily iron supplementation on health in children aged 4-23 months: a systematic review and meta-analysis of randomised controlled trials. | Micronutrient: Iron | 35 | RCTs |
| Petry 201637 | The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta- Analysis. | Micronutrient: Iron + zinc | 90 | RCTs or quasi-RCTs |
| Salam 201338 | Effectiveness of micronutrient powders (MNP) in women and children | Micronutrient: Micronutrient powders | 17 | RCTs |
| Sguaseero 201239 | Community-based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries (Review) | Community-based supplementary feeding | 8 | RCTs |
| Taylor-Robinson 201540 | Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance (Review) | Deworming | 45 | RCTs or quasi-RCTs |
Network meta-analysis, as an extension of conventional pairwise meta-analysis, allows for comparisons of interventions that have not been directly compared in head-to-head randomized clinical trials within a single analysis10,11. While network meta-analysis is new to MNCH, this technique has been endorsed by the World Health Organization (WHO) to support the development of intervention guidelines in global health11, with the past WHO guidelines on HIV drug and behavioral therapies and direct acting agents against hepatitis C having been formulated using network meta-analysis12–14. This study uses a systematic review and network meta-analysis to determine the comparative effectiveness across intervention domains in micronutrient supplements, food supplements, deworming, maternal education, and WASH interventions on HAZ and stunting for children aged 6–24 months living in LMICs.
The protocol for this study was registered on PROSPERO (CRD42018110449). The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension to network meta-analysis15.
Two-way sensitivity searches were conducted for this study. First, key MNCH articles, including Bhutta et al.16, and the Lancet 2013 umbrella review on evidence-based interventions2,17, were reviewed for relevant systematic reviews and trials. A hand-search of the bibliography of Bhutta et al.16 was done to identify relevant systematic reviews and trials, and searches were done on PubMed and the Cochrane Database of Systematic Reviews to identify additional reviews that were published after 2013. The list of published reviews relevant to this study is provided in Table 1.
As the second step, a full comprehensive search of literature was conducted from database inception up to September 17, 2019. The Cochrane Central Register of Controlled Trials, Embase, and MEDLINE were searched to identify relevant trials and any additional relevant reviews that were missed in the prior step (search terms are provided in Extended data, Supplementary Tables 1, 2, and 3)18. Hand searches were done on the reference lists from the relevant reviews identified to improve the sensitivity of this study’s search.
Table 2 summarizes the Population, Intervention, Comparator, Outcomes, and Study Design (PICOS) criteria used to guide the study selection for our systematic literature review. In brief, randomized clinical trials that assessed interventions’ comparative effectiveness on HAZ and/or stunting (a HAZ score of less than 2 SDs below the WHO Child Growth Standards median)19 for children aged 6 to 24 months living in LMICs. The intervention domains of focus included: micronutrient supplements, food supplements, deworming, maternal education, and WASH interventions. Non-English-language studies were excluded. Four reviewers (JJHP, ES, LD, and NEZ) independently reviewed all abstracts and proceedings identified in the systematic search. The same reviewers independently conducted the full-text review. Any discrepancies were resolved by discussion, and if a resolution could not be achieved, a fifth reviewer (KT) settled the disagreements.
Using a standardized data sheet in Microsoft Excel, four investigators (JJHP, ES, LD, and NEZ) independently extracted data for study characteristics, interventions used, participant characteristics at baseline, and outcomes from the final subset of eligible studies. Any discrepancies observed during data extraction were resolved by consensus achieved through discussion.
The network meta-analyses for this study were done using the Bayesian framework in R via the R2WinBUGS v14 package20,21. Bayesian models were performed according to the guideline of NICE Technical Support Document 2 (TSD2)41. Estimates of comparative effectiveness were measured using mean differences in HAZ with associated 95% credible intervals (95% CrI); and risk ratios (RRs) with associated 95% CrI for the stunting outcome. As heterogeneity was anticipated, random-effects network meta-analysis models were performed. In all models, an empirically informative heterogeneity prior distribution were used, as suggested by Rhodes et al.42 for continuous outcomes and Turner et al.43 for dichotomous outcome, to stabilize the estimation of heterogeneity in the face of low number of trials per comparison in the network. The model selection was informed by using the deviance information criterion (DIC) and the deviance-leverage plots that could help identify outlier(s) in terms of model fit, in accordance to the NICE TSD2 recommendations41.
For both HAZ and stunting, the primary analysis included both cluster and non-cluster randomized clinical trials (with the unit of randomization performed at the individual level). To adjust for clustering effects of the cluster trials, a conservative intracluster correlation coefficient of 0.05 was assumed and used inflated variances accordingly for the continuous outcome, and adjusted the sample sizes and the number of cases for the dichotomous outcome, following the principles recommended by Uhlmann et al.44 Sensitivity analyses were conducted for each outcome by using non-cluster randomized clinical trials only in the analyses. Full details of the statistical approaches are provided in the Extended data (Supplementary Table and figures file)18.
A total of 20,511 abstracts was found from our database searches and hand searches of the bibliography of published reviews (Figure 1). Of these, 1,094 studies underwent a full-text review with 96 papers reporting on 79 trials that met the inclusion criteria. The list of included studies is provided in the Extended data (Supplementary Table 4 for included and Table 5 for the list of excluded studies)18. Trial characteristics and participant characteristics of the included studies are provided in the Extended data, Supplementary Tables 6 and 718, respectively. In total, these trials comprised of 81,786 children who were randomized to 236 unique interventions (Figure 2). Of these trials, 22 were cluster trials with 2,990 clusters (53,057 children) randomized to 80 interventions. The majority of trials were conducted in African (n = 35) and South Eastern Asian (n = 25) countries with double blinding (i.e. blinding of participants and investigators; n = 40) being the most common blinding feature. Micronutrient supplementation was the most common intervention domain studied (n = 50 trials). Only a handful of these micronutrient trials compared interventions from other domains (food supplements: n = 11 trials46–56 and maternal education: n = 2 trial57,58). There were four cluster trials on WASH (WASH Benefits Bangladesh59, WASH Benefits Kenya60, SHINE61, and Shafique et al.62), and these trials also included intervention arms that consisted of food supplements (i.e. LNS) or multiple micronutrients (i.e. MMN).
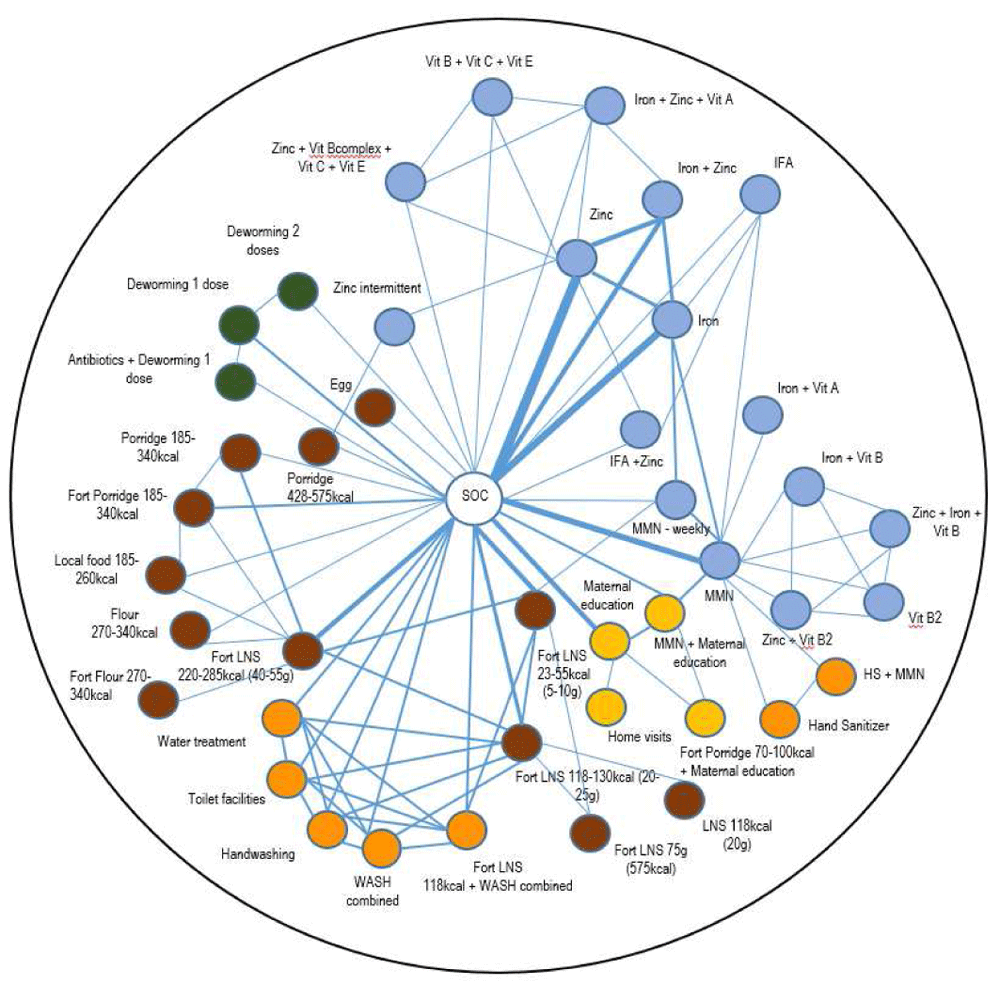
Each node (circle) represents an intervention, each line represents a direct comparison between interventions, with the lines with width representing the number of trials with the direct comparisons in question (i.e. thicker width represents a direct comparison with larger numbers of trials). The different intervention domains are indicated with the following colors: blue for micronutrient supplements; brown for food supplements; yellow for education and counseling interventions; green for deworming interventions; and orange for WASH interventions. Vit. vitamin; IFA, iron and folic acid; LNS, lipid-based nutrient supplements; Fort, fortification; MMN, multiple micronutrients; HS, hand sanitizer.
The network of evidence pertaining to the analysis of the outcome HAZ included 67 trials (69,223 children randomized to 220 intervention arms; Extended data, Supplementary Figure 1)18. Of these, 16 were cluster trials that randomized 1,440 clusters (36,032 children) to 62 intervention arms. Key results of the primary analysis that included both cluster and non-cluster randomized clinical trials are illustrated using a forest plot (Figure 3). Among micronutrient supplementations, iron + folic acid (IFA) (mean difference =0.08 95% CrI: 0.01, 0.15), and multiple micronutrients (MMN) (mean difference =0.06; 95% CrI: 0.01, 0.11) showed improvements in HAZ in comparison to standard-of-care. Iron (mean difference =0.03; 95% Crl: -0.02, 0.08) showed a trend towards HAZ improvement versus standard-of-care, but its credible intervals contained the null effect of 0. No food supplements showed improvements for HAZ versus standard-of-care. Similarly, no deworming interventions during the complementary feeding period or WASH interventions showed improvements in HAZ compared to standard-of-care.
The network diagram of the sensitivity analysis restricted to non-cluster randomized clinical trials for HAZ is provided in the Extended data (Supplementary Crosstable and Supplementary Tables and Figures)18. In comparison to standard of care, IFA showed results highly consistent with the primary analysis (mean difference =0.08; 95% CrI: 0.00, 0.16). MMN, on the other hand, did not show effectiveness over standard-of-care in the sensitivity analysis, but the trend was similar to the findings from the primary analysis (mean difference =0.05; 95% CrI: -0.03, 0.12). Similar to the primary analysis, no deworming and food supplements showed improvements in HAZ in comparison to standard-of-care. No WASH interventions were available for the sensitivity analysis, as it was limited to non-cluster trials only.
The network of evidence for the primary analysis of the stunting outcome consisted of 20 trials with 40,193 children randomized to 77 intervention arms (Extended data, Supplementary Figure 3)18. Of these, 12 were cluster trials with 1,608 clusters (33,660 children) randomized to 50 intervention arms. A forest plot for the comparative effects of interventions on stunting (RRs) is provided in Figure 4. Among micronutrient supplements, MMN (RR: 0.86, 95% CrI: 0.73, 0.98) demonstrated superiority over standard-of-care, whereas IFA (RR: 0.92, 95% CrI: 0.64, 1.23) did not reduce the risks of stunting relative to standard-of-care; however, intake of Iron (RR: 0.91; 95% Crl: 0.76, 1.06) showed trend towards reducing risks for stunting. For food supplements, fortified lipid-based nutrient supplements (LNS) containing 220–285 kcal (RR: 0.80, 95% CrI: 0.66, 0.97) and flour containing 270 – 340 kcal (RR: 0.73, 95% CrI: 0.51, 1.00) showed reduced risks of stunting versus standard-of-care. Among other intervention domains, compared to standard-of-care, Maternal education also showed a trend towards decreasing the risks of stunting (RR: 0.91, 95% CrI: 0.75, 1.08); but no deworming or WASH interventions showed reduced risks for stunting except for WASH combined + fortified LNS containing 118 kilocalories that had showed a trend towards reducing the risks of stunting (RR: 0.91, 95% CrI: 0.73, 1.10).
The network diagram of the sensitivity analysis for stunting is provided in the Extended data (Supplementary Figure 4)18. The results of the sensitivity analyses are provided in the Extended data, Supplementary Cross-table file (tab: “Sensitivity, Stunting”)18. We found several interventions that showed either reduced risks for stunting or trends towards reduced risks. For instance, relative to standard-of-care, MMN (RR: 0.78; 95% CrI: 0.59, 1.00) and fortified LNS 220–285 kcal (RR: 0.80; 95% Crl: 0.62, 1.00) showed reduced risks for stunting. Similarly, iron (RR: 0.89, 95% CrI: 0.74, 1.06) and flour 270–340 kcal (RR: 0.71; 95% Crl: 0.48, 1.02), showed trends towards reducing the risks of stunting versus standard-of-care, but their Crls contained the null effect of 1. No deworming interventions showed reduced risks for stunting over standard-of-care. Similar to the sensitivity analysis for HAZ, no WASH interventions were available.
In this study, systematic literature review and network meta-analysis were used to determine the comparative effectiveness of interventions for linear growth under the domains of micronutrients, food supplements, deworming, maternal education, and WASH interventions for LMIC-based children in the age group of 6 to 24 months. During the complementary feeding period life stage, micronutrient supplements such as IFA and MMN showed improvements for HAZ compared to standard-of-care, with iron showing some trends towards improved HAZ. Deworming, maternal education, food supplements, and WASH interventions, on the other hand, did not show improvements in HAZ versus standard-of-care. For stunting, food supplements of fortified LNS 220–280 kcal and flour 270–340 kcal showed reduced risks of stunting, with other interventions such as iron, MMN, and MMN combined with maternal education, demonstrating trends towards reduced stunting risks, in comparison to standard-of-care.
The key strengths of this study were the consideration of multiple intervention domains and the use of network meta-analysis. The approach undertaken for this study differed from previous reviews (Table 1) that have had limited scopes of single intervention or single intervention domains; these reviews have all used pairwise meta-analysis, thus being limited to trials and interventions that have only been directly compared to one another. The use of network meta-analysis allowed for consideration of a broader evidence base to estimate the comparative effectiveness of multiple interventions under multiple treatment domains. By incorporating statistical adjustments for clustering effects, this study was able to incorporate cluster randomized clinical trials that mostly did not report information on clustering effects (i.e. ICC or design effects) into the statistical analyses.
Nonetheless, the existing evidence base limited our analyses. The majority of the randomized clinical trial evidence base was confined to the micronutrient supplementation domain (n = 50), and the evidence base for intervention domains for food supplements, deworming, maternal education, and WASH being limited. This imbalance in intervention class could partly be explained by the narrow bounds in the population criterion (i.e. the age criteria of 6 to 24 months) of our PICOS criteria. For instance, there were a number of trials that recruited children using a wider age eligibility criterion (e.g. 6 months up to 5 years of age) that encompassed children in the complementary feeding life stage, but these trials were excluded since growth rates and determinants of children older than 24 months are different than children in the complementary feeding period age group. While this age restriction limited the number of eligible trials for our analysis, it is important to note that the population criteria was determined a priori before the screening was initiated for this systematic review since we recognized that growth determinants and rates can vary substantially for children between these ages63.
This study has shown that the existing evidence base on interventions aimed to improve linear growth in children during the complementary feeding period is limited and inconsistent. Generally, investigation of interventions outside of the domain of micronutrient supplements was limited. There were only two trials on deworming64,65 and four WASH trials59–62 reporting on linear growth outcomes in our analyses. There were eight trials under the maternal education domains57,58,62,66–70, but the components and the delivery of these educational interventions varied considerably between these trials. For food supplement trials, poor adherence and household food insecurity that may promote family sharing could influence why these interventions did not show improvements in linear growth. Moreover, the food supplements in these trials were all based on a single type of food, so children may have refused to consume as they have become tired of consuming same type of food over time. There were no trials that investigated nutritional strategies that aimed to improve dietary diversity in order to improve linear growth in children during this life stage, nor were there any trials that aimed to address household food insecurity.
A previous report from Bangladesh has shown that the taste of LNS is generally acceptable, and at least in shorter-term, adherence to LNS was high and sharing of these food supplements could be minimized in the household71. However, acceptability and adherence to LNS in other settings are not clear; the long-term acceptability and adherence to LNS or other types of food supplements that consist of a single food on daily basis over long-term are also questionable. Additionally, our analysis did not show that supplementing children with high caloric food supplements result in improved linear growth when compared to standard of care or to other lower caloric food supplements. Aside from previously described issues associated with tolerability of nutritional supplementation that may be exacerbated by high caloric formulations, it is possible that in households with food insecurity, caregivers may choose to share these supplements with other children or members of the household who are not enrolled in the trial. Understanding compliance and the influence that this may have on our analyses was not possible, as we found that compliance was usually not measured or reported in the included food supplement trials72–76.
Our findings identified several directions for future research. There is a need to combine interventions across multiple domains as a package. Instead of singling out interventions from one domain, there is a need for comparisons between different packaged interventions because a combined set of interventions will likely result in the greatest improvements in linear growth. Strategies should consider local contexts and challenges. The feasibility of conducting trials that incorporate food supplement strategies aimed to improve dietary diversity and address household food insecurities is undoubtedly difficult. However, it is important to recognize these factors will be important for long-term acceptability and adherence to food interventions, and interventional strategies that incorporate diverse local foods will have higher acceptability and adherence in the long run. Trials with longer follow-up are also needed, as the median follow-up among the trials included for this review was only 6 months. Lastly, there is a need for more innovative trial approaches77. The majority of the trials identified for this review used a conventional trial approach with a fixed sample size design, where the assessment of interventions occurred only after the number of participants recruited reached the sample size target. It is important to recognize that such approach can be inefficient, and adopting adaptive trial design approaches that allow for pre-specified modifications, with the decision being made based on accumulating trial data may improve both the efficiencies of the trial evaluation in this avenue of research78.
All data underlying the results are available as part of the article and no additional source data are required.
Open Science Framework: Interventions to improve linear growth during complementary feeding period for children aged 6–24 months living in low- and middle-income countries: a systematic review and network meta-analysis. https://doi.org/10.17605/OSF.IO/XU79N18.
This project contains the following extended data:
Complementary feeding period NMA - Supplementary Tables and Figures - v2.0
Appendix 1. Literature search. (Contains Supplementary Tables 1–3.)
Appendix 2. List of included and excluded studies after full-text review. (Contains Supplementary Tables 4 and 5.)
Appendix 3. Details of the evidence base. (Contains Supplementary Tables 6 and 7.)
Appendix 4. Network for HAZ (Contains Supplementary Figures 1 and 2.)
Appendix 5. Network for stunting outcome. (Contains Supplementary Figures 3–9.)
Complementary feeding period NMA - Supplementary Crosstable - v1.0
Open Science Framework: PRISMA checklist for “Interventions to improve linear growth during complementary feeding period for children aged 6–24 months living in low- and middle-income countries: a systematic review and network meta-analysis.” https://doi.org/10.17605/OSF.IO/XU79N18.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
JJHP, KT, and EJM conceptualized and designed the study. JJHP, OH, ES, LD, NEZ, JS, RTL, KT, and EJM acquired, analyzed, and interpreted data. JJHP drafted the manuscript. All authors critically revised the manuscript for important intellectual content. JJHP, OH, KT, and EJM did the statistical analysis. EJM obtained funding. JS, RTL, KT and EJM provided administrative, technical, or material support. JS, RTL, KT, and EJM supervised the study.
KT and EJM had full access of all of the data in the study. EJM was responsible for the integrity of the data, accuracy of the data analysis, and the final decision to submit for publication.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nutrition, Epidemiology, Clinical trials in LMIC.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Systematic literature review and network meta-analysis.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: infectious disease, nutrition, trials in LMIC settings
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 24 Sep 20 |
read | ||
|
Version 1 14 Nov 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)