Keywords
Pregnancy, low- and middle-income countries, network meta-analysis, evidence synthesis, preterm, birthweight, birth outcomes
Pregnancy, low- and middle-income countries, network meta-analysis, evidence synthesis, preterm, birthweight, birth outcomes
Table 1 - studies related to financial incentives and flu immunization have been removed as they are not relevant.
Methods section - the first sentence under search strategy and selection criteria has been updated to specify the scope of research further.
Results section - added more details on maternal education.
Discussion section - first sentence has been updated to keep description of intervention domains consistent with the introduction.
References section - 4 references removed in order to be consistent with Table 1.
See the authors' detailed response to the review by R Rima Jolivet
See the authors' detailed response to the review by Sonali Kochhar
See the authors' detailed response to the review by Elizabeth M. McClure
Despite global substantial progresses that have been made towards improving maternal, newborn, and child health (MNCH) in the last two decades, adverse birth outcomes such as preterm birth and low birthweight still remain as an important global health challenge, particularly in low- and middle-income countries (LMIC)1–3. Determinants of these challenges are multifaceted4–7. Pregnant women in LMICs have a higher risk of nutritional deficiencies, stemming from physiological changes that involve fetal development and growth resulting in an increased demand for nutrients4,5. Poor water, sanitation, and hygiene (WASH) control can also increase likelihood for infectious diseases, including intestinal worm infections that may contribute to conditions, such as anemia, which negatively affects fetal survival and growth6,8,9. Poor maternal health during pregnancy is associated with preterm birth (<37 gestation weeks) and low birthweight (<2500 g), and these adverse birth outcomes are associated with adverse neonatal events, such as respiratory distress syndrome, neurocognitive impairment, poor linear growth (stunting), and overall mortality1,2,10,11.
Several reviews have aimed to assess the effectiveness of various promising interventions for pregnant women (Table 1). Despite the extensive research conducted to date, the comparative effectiveness of interventions remains unclear across different domains, such as micronutrients, balanced energy protein supplements, maternal education, deworming, and WASH. Few clinical trials have directly compared interventions across domains. Rather, the majority of clinical trials has only compared interventions within a domain. Similarly, most summaries of the evidence for pregnancy interventions have used traditional pairwise meta-analysis, allowing only for the quantitative assessment of a single intervention versus a comparator. Thus far, no attempts have been made to synthesize the evidence indirectly in order to make quantitative comparison of interventions that have not been directly compared in studies.
| Review ID | Title | Interventions | No of studies | Types of studies included |
|---|---|---|---|---|
| Imdad 201120 | Effect of balanced protein energy supplementation during pregnancy on birth outcomes | Balanced protein energy supplements | 11 | RCTs and quasi-RCTs |
| Imdad 201221 | Maternal Nutrition and Birth Outcomes: Effect of Balanced Protein-Energy Supplementation | Balanced protein energy supplements | 16 | RCTs and quasi-RCTs |
| Liberato 201322 | Effects of protein energy supplementation during pregnancy on fetal growth: a review of the literature focusing on contextual factors | Balanced protein energy supplements | 20 | RCTs, quasi-RCTs, and observational study |
| Stevens 201523 | The effect of balanced protein energy supplementation in undernourished pregnant women and child physical growth in low- and middle-income countries: a systematic review and meta-analysis | Balanced protein energy supplements | 7 | RCTs, quasi-RCTs, and observational study |
| Buppasiri 201524 | Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes | Calcium | 25 | RCTs and cluster-RCTs |
| Hofmeyr 201425 | Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems | Calcium | 13 | RCTs |
| Salam 201526 | Effect of administration of antihelminthics for soil-transmitted helminths during pregnancy | Deworming | 4 | RCTs |
| Lassi 201327 | Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes | Folic acid | 31 | RCTs and cluster-RCTs |
| Yang 201128 * | Review of fortified food and beverage products for pregnant and lactating women and their impact on nutritional status | Fortified products | 14 | RCT, quasi-RCT |
| Pena-Rosas 200929 | Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy | Iron; Iron + folic acid | 49 | RCTs and quasi-RCTs |
| Suchdev 201530 | Multiple micronutrient powders for home (point-of-use) fortification of foods in pregnant women (Review) | Multiple micronutrient powders | 2 | RCTs |
| Haider 201731 | Multiple-micronutrient supplementation for women during pregnancy | Multiple micronutrient supplements | 19 | RCTs |
| Imhoff-Kunsch 201232 | Effect of n-3 Long-chain Polyunsaturated Fatty Acid Intake during Pregnancy on Maternal, Infant, and Child Health Outcomes: A Systematic Review | N-3 long chain polyunsaturated fatty acid | 15 | RCT |
| Thorne-Lyman 2012A33 | Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta- analysis | Vitamin A | 17 | RCTs |
| De-Regil 201634 | Vitamin D supplementation for women during pregnancy | Vitamin D | 15 | RCTs and quasi-RCTs |
| Perez-Lopez 201535 | Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta- analysis of randomized controlled trials | Vitamin D | 13 | RCTs |
| Thorne-Lyman 2012B36 | Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis | Vitamin D | 5 | RCTs |
| Ota 201537 | Zinc supplementation for improving pregnancy and infant outcome | Zinc | 21 | RCTs |
| Goudet 201938 | Nutritional interventions for preventing stunting in children (birth to 59 months) living in urban slums in low-and middle-income countries (lmic) | Nutrient supplementation and Education | 15 | RCTs, quasi-RCTs, non- RCTs, controlled before- and-after, and interrupted time series |
Recognizing the paucity of direct head-to-head randomized clinical trials (RCTs) between existing interventions, a network meta-analysis can be used to summarize the entirety of evidence for pregnancy interventions. A network of interventions connected via the comparisons that have been made in head-to-head trials can be constructed, and where there is a path from one intervention to another, these interventions can be compared indirectly via some common comparators12–16. In addition, where both direct and indirect evidence exists, the indirect evidence can be used to strengthen the inferences for the particular comparison. This is particularly important for pregnancy interventions because many head-to-head trials of active interventions have limited sample sizes. Furthermore, network meta-analysis allows us to simultaneously analyze all potential treatment options and make full use of the available evidence within a single analysis.
The purpose of this study was to assess the comparative effectiveness across intervention domains in micronutrient supplements, balanced energy protein supplements, deworming, maternal education, and WASH interventions using network meta-analysis. Effectiveness of interventions are determined by the following outcome indicators: preterm birth, low birthweight, and birthweight for LMIC-based pregnant women.
Our analysis and report was designed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension to network meta-analysis17. The protocol for this study is registered on PROSPERO (CRD42018110446).
The scope of our research study and the corresponding search strategy was developed after first reviewing the papers published in the Lancet’s 2013 Maternal and Child Nutrition series1,18, including the umbrella review on evidence-based interventions by Bhutta and colleagues2, for an overview of the literature. Specifically, we reviewed the bibliography of Bhutta et al. 20132 for relevant systematic reviews, global health guidelines, and LMIC-based trials. We also performed hand searches on PubMed and the Cochrane Database of Systematic Reviews for reviews that have been published after 2013. The list of published reviews relevant to this study is provided in Table 1.
For our systematic literature search, the following databases were searched from inception to September 17, 2019: the Cochrane Central Register of Controlled Trials, Embase, and MEDLINE (Extended data, Supplementary Tables 1–3)19. In addition to database searches, we included the relevant trials identified from bibliographies of prior reviews (Table 1). Table 2 describes the PICOS criteria used to guide the study selection. We included LMIC-based RCTs on interventions related to the domains of micronutrient supplements, balanced energy protein (i.e. food supplementation) supplements, deworming, maternal education, and WASH; the outcomes of interest were preterm birth (<37 weeks of gestational age), low birthweight (<2500 grams), and birthweight (continuous). We excluded non-English language studies.
A paired group of four reviewers (JJHP, ES, MZ, and LD) independently reviewed all abstracts and proceedings identified in the literature searches. JJHP and ES worked in one pair, while MZ and LD worked in another pair. The same paired team independently reviewed abstracts potentially relevant in full-text. If any discrepancies occurred between the studies selected by the two investigators, a third investigator (KT) provided arbitration.
Using a standardized data sheet, a paired group of four reviewers (JJHP, ES, MZ, and NEZ) independently extracted data for study characteristics, interventions used, patient characteristics at baseline, and outcomes from the final list of selected eligible studies. Any discrepancies observed between the data extracted by the four extractors were resolved by consensus through discussion. Primary outcomes were dichotomous, consisting of preterm birth and low birthweight. Our secondary endpoint was the continuous outcome of birthweight. We preferentially extracted intention-to-treat outcomes.
We performed our analyses within the Bayesian framework in R using the R2WinBUGS v14 package39,40. Bayesian models were performed according to the National Institute for Health and Care Excellence (NICE) in their Technical Support Document 2 (TSD2)41. The network diagrams with respective to the analyzed outcome can be seen in Extended data, Supplementary Figures 1–619. Estimates of comparative effectiveness are measured using risk ratios (RRs) with associated 95% credible intervals (95% CrI) for preterm birth and low birthweight, and mean differences and the associated 95% CrI for birthweight. We performed random-effects network meta-analysis models using an empirically informative priors for the heterogeneity variance, as suggested by Rhodes et al.42 for mean birthweight and Turner et al.43 for preterm birth and low birthweight. This was done to stabilize the estimation of heterogeneity in the face of low number of trials per comparison in the network. Our model selection was informed by the deviance information criterion (DIC) and the deviance-leverage plots that could help identify outliers or lack of model fit.
As our primary analysis, we included both cluster and individually randomized (non-cluster) clinical trials. To adjust for clustering effects of the cluster trials, we adjusted the sample sizes and number of cases for preterm birth and low birthweight and inflated variances for mean birthweight to account for clustering effects of the cluster trials, as recommended by Uhlmann et al.44, assuming a conservative intra-cluster correlation coefficient (ICC) value of 0.05. For each outcome, we performed sensitivity analyses by excluding cluster randomized clinical trials where the analyses were limited to individually randomized clinical trials only.
We identified 5,297 abstracts from our database searches and hand searches of the bibliography of the published reviews (Figure 1). Of these, 377 studies underwent a full-text review, and 132 papers reporting on 101 trials met our inclusion criteria. In total, these trials included 206,531 pregnant women that were randomized to 245 unique interventions (Figure 2). The list of included and excluded studies (Extended data, Supplementary Tables 4 and 5)19, as well as the trial and patient characteristics of the included studies (Extended data, Supplementary Tables 6 and 7)19 are provided in the Extended data. Geographically, most trials were conducted in South Eastern Asian (n = 38 trials) and African (n = 26 trials) countries, with individual randomization (i.e. non-cluster trials, n = 85 trials) and double blinding (n = 52 trials) being the most common methodological features. Micronutrient supplements was the most common intervention domain that was investigated (n = 79 trials); only a few of these micronutrient trials compared interventions from other domains, such as balanced energy protein supplements (n = 15 trials) and deworming (n = 6 trials). Maternal education was captured in a few trials (n=3) where the education component was in the form of counseling, lifestyle education, and participatory learning action (PLA) with government-mandated women’s groups. PLA involved awareness of the problem of LBW and malnutrition, and strategies to overcome barriers to improved health and nutrition. There were no WASH trials reporting on the analyzed birth outcomes.
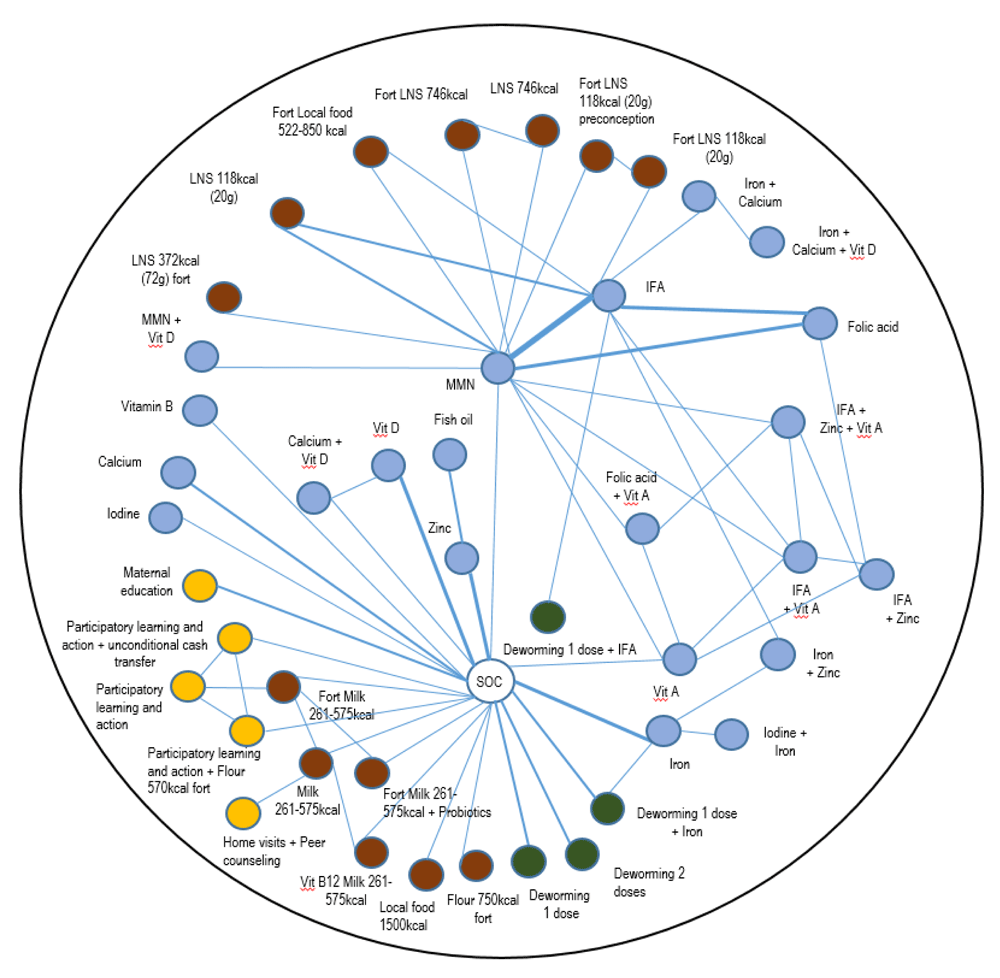
Each node (circle) represents an intervention, each line represents a direct comparison between interventions, with the lines with width representing the number of trials with the direct comparisons in question (i.e. thicker width represents a direct comparison with larger numbers of trials). The different intervention domains are indicated with the following colors: blue for micronutrient supplements; brown for balanced energy protein supplements; yellow for education and counseling interventions; and green for deworming interventions. Vit, Vitamin; IFA, iron and folic Acid; LNS, lipid-based nutrient supplements; Fort, fortification; MMN, multiple micronutrients.
In most trials, interventions were provided to pregnant women from enrollment until delivery (n = 87 trials). These trials generally involved women who were in the later part of their gestational age. For instance, only 5 trials enrolled women from or before conception (Owens46, The women First Trial47, CAP Trial48, PRECONCEPT49, and Brabin50), while the majority of trials recruited women who were in the later trimesters, such as the 2nd and 3rd trimesters (n = 69 trials).
The preterm birth network (Extended data, Supplementary Figure 1)19 included 64 trials consisting of 85,546 pregnant women randomized to 152 intervention arms (ten cluster trials consisting of 1,998 clusters and 20,218 pregnant women). From the primary analysis, that included both cluster and non-cluster randomized clinical trials, only few interventions showed superiority over standard-of-care for preterm birth (Figure 3). For instance, compared to standard-of-care, intake of 1500 kcal of local food per day showed an RR of 0.36 (95% CrI: 0.16, 0.77) and calcium showed an RR of 0.76 (95% CrI: 0.56, 0.99). Other micronutrient supplements such as folic acid (RR: 0.71, 95% CrI: 0.43, 1.09), iron (RR: 0.70, 95% CrI: 0.47, 1.01), zinc (RR: 0.67, 95% CrI: 0.41, 1.04), and multiple micronutrients (MMN) (RR: 0.70, 95% CrI: 0.45, 1.02) showed a trend towards lower preterm birth risks compared to standard-of-care, but their Crls overlapped the null effect of 1.00. In comparison to standard-of-care, no balanced energy food supplements, other than 1500 kcal of local food showed reduction in preterm birth risks, and neither did maternal education interventions (e.g. participatory learning action51).
The mean birthweight network (Extended data, Supplementary Figure 3)19 included of 81 trials that consisted of 130,315 pregnant women randomized to 196 intervention arms. Of these 81 trials, 14 were cluster trials that randomized 1,354 clusters (57,483 pregnant women) to 35 intervention arms. The results of the network meta-analysis on mean birthweight can be found in Figure 4. Among the micronutrient supplementation domain, compared to standard-of-care, MMN (mean difference: 0.27 kg; 95% CrI: 0.09, 0.45 kg), folic acid (mean difference: 0.21 kg; 95% CrI: 0.00, 0.42 kg), iron (mean difference: 0.18 kg; 95% CrI: 0.02, 0.34 kg), and iron + folic acid (IFA) (mean difference: 0.18 kg; 95% CrI: 0.00, 0.36 kg) showed improvements in birthweight. Among the balanced energy food supplements, unfortified lipid-based nutrient supplements of 20 grams (LNS20) showed improvements in birthweight compared to standard-of-care (mean difference: 0.27 kg; 95% CrI: 0.03, 0.51 kg). Deworming and maternal education interventions did not improve mean birth weight; for instance, in comparison to standard-of-care, a single dose of deworming showed a mean difference of 0.02 kg (95% CrI: -0.16, 0.19 kg) and maternal education showed a mean difference of 0.07 kg (95% CrI: -0.27, 0.40 kg).
The low birthweight network (Extended data, Supplementary Figure 5)19 consisted of 67 trials, with 84,675 patients randomized to 160 intervention arms (eleven cluster trials consisting of 792 clusters and 9,512 pregnant women). The results on low birthweight (kg) outcome can be found in Figure 5. High caloric local food intervention (1500 kcal per day) reduced the risk of low birthweight (RR: 0.17; 95% CrI: 0.09; 0.29). There was a trend towards reduced risks of low birthweight for other interventions such as calcium (RR: 0.87; 95% CrI: 0.69; 1.07), MMN (RR: 0.73; 95% CrI: 0.49, 1.07), and LNS20 (RR: 0.65; 95% CrI: 0.39, 1.07), fortified LNS20 (RR: 0.72, 95% CrI: 0.48, 1.03), but their 95% CrI contained the null effect of 1.00. A single dose of deworming during pregnancy did not show reduction in low birthweight when compared to standard-of-care (RR: 1.15, 95% CrI: 0.83, 1.58).
For all three outcomes, the results from the sensitivity analyses of studies limited to non-cluster randomized clinical trials can be found in the Extended data (Supplementary cross table excel file: Sensitivity Preterm, LBW, and Birthweight tabs)19. As fewer studies were available for the sensitivity analysis, the CrIs for many comparisons became wider, but the direction and the magnitude of comparative effects remained relatively stable. For instance, there were no individually randomized trials that evaluated the effectiveness of high caloric (1500 kcal per day) local food. In terms of micronutrient supplementation, MMN (mean difference: 0.10 kg; 95% CrI: 0.00, 0.20 kg) and iron (mean difference: 0.09 kg; 95% CrI: 0.02, 0.16 kg) improved mean birthweight by a small margin compared to standard-of-care. Similarly, unfortified lipid-based nutrient supplements (LNS20) did not improve mean birthweight to a great extent compared to standard-of-care (mean difference: 0.13 kg; 95% CrI: 0.01, 0.24 kg). Furthermore, in comparison to standard-of-care, maternal education (mean difference: 0.04 kg; 95% CrI: -0.13, 0.20 kg) and one dose of deworming (mean difference: 0.01 kg; 95% CrI: -0.04, 0.06 kg) did not have any effect on mean birthweight. As far as preterm birth is concerned, MMN (RR: 0.58; 95% CrI: 0.31, 1.00) showed a trend towards lower preterm birth risks compared to standard-of-care, but their Crls overlapped the null effect of 1.00.
In this study, we used network meta-analysis to compare the effectiveness of interventions across several domains consisting of micronutrient supplements, balanced energy protein supplements, deworming, maternal education, and WASH interventions that can be provided to pregnant women living in LMICs. Several micronutrient supplements demonstrated decreased risks for preterm birth and/or improve mean birthweight, compared with standard-of-care for pregnant women. For example, MMN interventions showed reduction in preterm birth risks and improved mean birthweight. In comparison to standard-of-care, IFA, calcium, iron, and zinc also demonstrated a trend towards decreasing preterm birth risks. However, the evidence for other intervention domains were limited. For instance, among balanced energy protein supplements, only consumption of 1500 kcal of local food supplement lowered the risks of preterm birth and low birthweight; and only unfortified LNS 20 demonstrated improvement in mean birthweight. Nevertheless, these findings pertaining to balanced energy protein supplements corresponded to only three trials in the study52–54. There was a limited number of trials available for maternal education and deworming intervention; no WASH trials reporting on preterm birth and birthweight outcomes were available.
The main strength of this study was the use of network meta-analysis to assess the effectiveness of different interventions from a large network of evidence compared to standard-of-care. Unlike previous reviews that have focused on one intervention within a single domain, we used a broad evidence base that included multiple interventions from different domains. As well, appropriate statistical adjustments were made for clustering effects of cluster randomized clinical trials to enable the convergence of cluster and non-cluster trials for our network meta-analysis. Nevertheless, the existing evidence base limited our analyses. Few trials reported low birthweight, and the majority of randomized clinical trial evidence base was confined to a single domain of micronutrient supplementation. Another possible limitation was that there was notable variation in the enrollment of pregnant women in terms of trimesters and gestational age. While we did not find that time of enrollment relative to gestational age was a treatment effect modifier in our analyses, we acknowledge that this variation may have introduced heterogeneity in our meta-analyses. Prior evidence has also demonstrated mixed evidence as to whether the time at which treatment is initiated influences overall treatment efficacy, and this varies by treatment type25,31,37. Lastly, our assumption of a conservative ICC (0.05) may also have affected the results. However, this was necessary in order to assess for the entire evidence base of interventions for pregnancy, as most cluster randomized trials did not report ICC for each outcome.
Despite these limitations, the findings of this study were generally similar to that of other existing reviews. For instance, among the micronutrient supplements, other reviews have shown that iron (RR=0.82, 95%Crl 0.72, 0.94)55 and MMN (RR=0.88, 95%Crl 0.85, 0.90)31 reduced the risks of low birthweight versus standard-of-care. Moreover, calcium (RR=0.76, 95%Crl 0.60, 0.97)25 and zinc (RR=0.86, 95%Crl 0.76, 0.97)37 supplements reduced the risks of preterm birth, and we have found that intake of combined MMN reduced the risks of preterm birth and improved mean birthweight. Similar to this study, Salam26 found no improvements in low birthweight and preterm birth for deworming versus standard-of-care. There were no reviews on WASH available that looked at the role of WASH interventions on birthweight and preterm birth.
Our findings identified several directions for future research. First, there is a need to combine interventions that consist of compelling and evidence-based interventions of different domains as a package, moving away from a reductionist approach that is reflected in the majority of clinical trials conducted so far. Instead of a singling out interventions from one domain, there is a need for more evidence of packaged interventions because a combined set of interventions will likely result in the greatest improvement for adverse birth outcomes. Second, more research is needed to assess the longevity of interventions and its effectiveness across multiple life stages. For instance, only 17 out of 101 trials conducted follow-ups of women after birth delivery into the post-partum period. It is also important to note that the median follow-up of pregnant women beyond delivery was 8 weeks and only three trials23,56,57 conducted follow-ups with women and their newborns up to 6 months of age.
Overall, we identified a number of interventions for pregnancy with clear and compelling supportive evidence for effectiveness for preventing adverse birth outcomes. In midst of the World Health Organization’s Global Nutrition Targets 202558, which focuses on improving maternal, infant, and young children nutrition, national and local MNCH programs should consider adopting and adapting effective interventions identified in this review based on their local resource availability and program priorities. This may provide an opportunity to evaluate the benefits of these interventions in routine practice for pregnancy, and a step towards reaching the 2025 Global Nutrition Target of reducing the global prevalence of low birthweight by 30%59.
All data underlying the results are available as part of the article and no additional source data are required.
Open Science Framework: Interventions to improve birth outcomes of pregnant women living in low- and middle-income countries: a systematic review and network meta-analysis.
https://doi.org/10.17605/OSF.IO/JK3AQ19.
This project contains the following extended data:
Pregnancy NMA - Supplementary tables and figures - v2.0:
Appendix 1. Literature search strategy. (Contains Supplementary Tables 1–3.)
Appendix 2. Details of statistical analyses.
Appendix 3. List of included and excluded studies are full-text review. (Contains Supplementary Tables 4 and 5.)
Appendix 4. Details of the evidence base. (Contains Supplementary Tables 6 and 7.)
Appendix 5. Bias Assessment. (Contains Supplementary Table 8.)
Appendix 6. Intervention networks for birth outcomes (Supplementary Figures 1–6.)
Appendix 7. Primary analysis leverage and consistency plots. (Supplementary Figures 7–12.)
Appendix 8. Sensitivity analysis forest plots, non-cluster trials. (Supplementary Figures 13–15.)
Appendix 9. Sensitivity analysis leverage plots, non-cluster trials. (Supplementary Figures 16–18.)
Pregnancy NMA - Supplementary crosstables - v1.0
Open Science Framework: PRISMA checklist for “Interventions to improve birth outcomes of pregnant women living in low- and middle-income countries: a systematic review and network meta-analysis.” https://doi.org/10.17605/OSF.IO/JK3AQ19.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
JJHP, KT, and EJM conceptualized and designed the study. JJHP, OH, ES, MZ, LD, NEZ, RTL, KT, and EJM acquired, analyzed, and interpreted data. JJHP drafted the manuscript. All authors critically revised the manuscript for important intellectual content. JJHP, OH, KT, and EJM did the statistical analysis. EJM obtained funding. KT and EJM provided administrative, technical, or material support. KT and EJM supervised the study. KT and EJM had full access to all of the data in the study. EJM was responsible for the integrity of the data, accuracy of the data analysis, and the final decision to submit for publication.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Public health, maternal health, measurement, qualitative and quantitative research.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Maternal Immunization, Maternal and Child Research, Adverse Maternal and neonatal outcomes
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology, maternal and newborn health in LMICs
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Public health, maternal health, measurement, qualitative and quantitative research.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 24 Sep 20 |
read | ||
|
Version 1 08 Nov 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)