Keywords
Soil-transmitted helminths, WHO guidelines, morbidity control, NTD Modelling
This article is included in the 2030 goals for neglected tropical diseases collection.
Soil-transmitted helminths, WHO guidelines, morbidity control, NTD Modelling
Minor edits were made to clarify the following issues highlighted by Reviewer 2:
1) Achieving 75% community-wide treatment is especially important to reach the morbidity target for hookworm.
2) Elimination of transmission can be reached in low-transmission settings. The study on which this insight is based used the basic reproductive number as a measure of transmission intensity, not prevalence.
3) All simulation studies have been run for the different STH species (Ascaris lumbricoides, Trichuris trichiura, and hookworm species). Insights are, therefore, species-specific.
4) The results and conclusion with regard to the importance of WASH are based on simulations for the impact of sanitation and hygiene measures in isolation and in combination.
See the author's detailed response to the review by Suzy J. Campbell
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the views of the World Health Organization. Publication in Gates Open Research does not imply endorsement by the Gates Foundation.
Soil-transmitted helminth (STH) infections are caused by several species of parasitic worms that are transmitted by eggs present in human faeces, which contaminate the soil in areas with poor sanitation. STHs cause some of the most common infections, with about 1.5 billion people infected worldwide1. The three main STHs are roundworm (Ascaris lumbricoides), whipworm (Trichuris trichiura) and hookworm (Necator americanus and Ancylostoma duodenale). STHs reduce the nutritional status of infected individuals1. In particular, infected children can be affected by reduced physical fitness and impaired growth and cognitive development1. Hookworm infection in women of reproductive age (WRA) can lead to severe anaemia1. Infections with A. lumbricoides and hookworms can be treated effectively with benzimidazole drugs (albendazole, mebendazole). However, benzimidazoles are less effective against T. trichiura. Dual treatment with albendazole and ivermectin increases treatment efficacy for T. trichiura2–4. Currently, albendazole and mebendazole are donated to the World Health Organization (WHO) for distribution to affected populations.
The WHO has announced morbidity control as the main public health target for STHs to be achieved by 2030. According to the most recent WHO guidelines, morbidity control is defined as <2% prevalence of medium-to-high intensity (M&HI) infections in preschool-age children (preSAC) and school-age children (SAC). WHO treatment guidelines advise preventive chemotherapy (PC) by mass drug administration (MDA) to achieve morbidity control. Previously, WHO recommended school-based PC without including adults. The most recent guidelines recommend PC targeted at preSAC, SAC and WRA. The frequency of PC is based on the prevalence of STH infections in SAC prior to the start of treatment (see decision tree in Figure 1 for WHO guidelines up until 2019). The recommended PC coverage is 75% in all targeted populations.
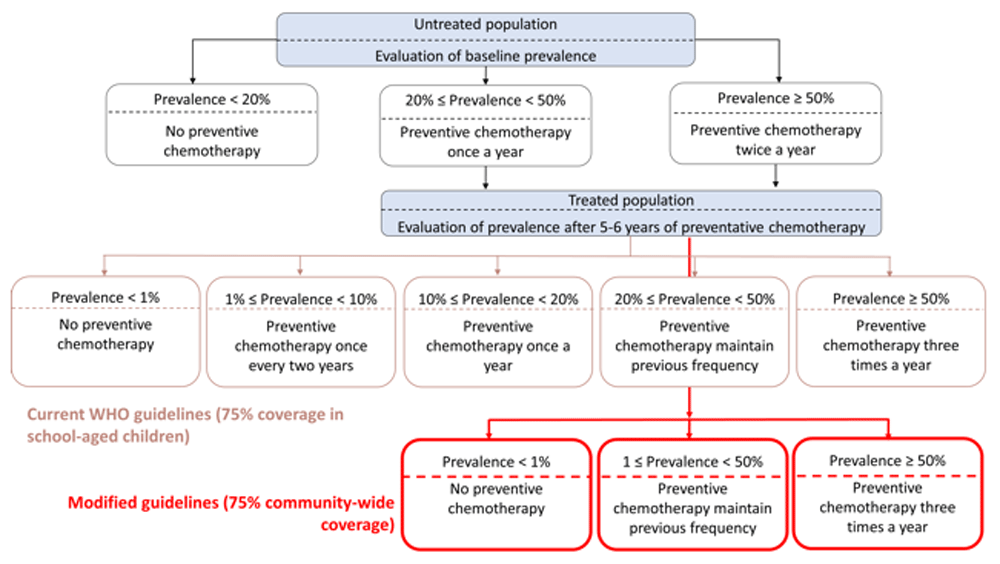
The bright red boxes represent the modified guidelines assuming 75% community-wide coverage (pre-SAC, SAC, and adults to replace the middle row of boxes that represent the current guidelines. This figure has been adapted from Figure 1 in 5 under a CC-BY 4.0 license.
Mathematical models of STH transmission dynamics and the impact of interventions have been developed to evaluate optimal treatment strategies for achieving the WHO goals. The Neglected Tropical Diseases Modelling Consortium (NTDmc) funded by the Bill and Melinda Gates Foundation brings together research groups from multiple scientific institutions working on neglected tropical diseases (NTDs), including STHs. Modelling groups based at Erasmus Medical Center (EMC) in Rotterdam and Imperial College London (ICL) have led the recent work on STHs. A model comparison was carried out for the EMC and ICL STH models6. Moreover, joint papers evaluating WHO treatment guidelines and monitoring and evaluation strategies have been published5,7. Predictions have been made for each STH species to account for inter-species variability. In these predictions it is important to note that models predict true prevalence while surveillance data make predictions that are sensitive to the diagnostic method employed. The predictions of both models are largely comparable, although the EMC model is more optimistic about the additional impact of community-wide vs. targeted (pre-SAC, SAC and WRA) deworming, which can be readily explained by differences in assumptions about how rates at which individuals contaminate the environment vary with age.
The WHO has proposed new goals for NTDs, including new control targets for STHs in the year 2030. Using insights from recent modelling work we discuss the feasibility of reaching the morbidity target following current guidelines and the risks that need to be mitigated to maintain the target (summary in Table 1).
Our modeling and epidemiological data analyses have shown that the current WHO treatment guidelines are sufficient to achieve the 2020 morbidity target in settings where the prevalence was moderate (20% to 50% in SAC) prior to the start of PC5. For higher prevalence settings, community-wide PC and/or targeting of WRA will be necessary to achieve the morbidity target, especially for hookworm5,8, and/or dual therapy with albendazole and ivermectin for T. trichiura5,9. Implementing PC twice-yearly also increases chance to achieve the morbidity target for STH5. Scaling down or stopping PC as per WHO treatment guidelines is very likely to lead to resurgence of infection to levels above the morbidity target, unless transmission conditions are addressed with water, sanitation and hygiene (WASH)10 or elimination of transmission (EOT) is achieved5. If this is not feasible, PC needs to be sustained10. Impact assessments potentially need to be repeated at regular intervals. Accurate measurements of access and compliance to PC remain essential to evaluate and sustain achievement of the targets. It is also important to note the poor sensitivity of Kato-Katz at low prevalence (models predict true prevalence).
Geospatial analyses of recent large-scale epidemiological studies of STH prevalence11 show that prevalence heterogeneity is considerable within PC implementation units. Our simulations suggest that to evaluate PC impact, a sufficient number of villages should be sampled in each implementation unit7 for an accurate assessment of the prevalence (number of villages depending on geospatial variation).
The indicator for the morbidity target will be measured with Kato-Katz (or any other validated quantitative technique). However, the number of slides/samples used strongly affects the measured prevalence12,13. PCR methods, although expensive at present, are a much more sensitive diagnostic at low prevalence. The indicator threshold would be more meaningful if linked to a standardized diagnostic procedure, or to the true prevalence of infection from which thresholds for specific diagnostic procedures and sampling designs can be derived.
Current egg counting methods suffer from considerable measurement error, which is compounded by high variation in egg density between and within persons over time13–15, meaning that an observed prevalence of M&HI can be well above the 2% target by chance. This is further compounded by an increase in inter-individual variation in egg counts as infection prevalence goes down during PC (likely due to systematic non-access to PC). Further, modelling suggests that “prevalence of any infection in preSAC and SAC” combined with a higher target threshold is a more informative indicator (higher positive predictive value) for meeting the morbidity target and would require a smaller sample size because of a higher statistical power7.
The 2030 morbidity target will be achievable in some countries. The frequency and duration of PC and implied resources depend on baseline prevalence and achieved coverage plus patterns of individual compliance to treatment5.
Treatment guidelines will lead to the achievement of the target in some communities, but not in all5. Current WHO guidelines do not call for treatment in low prevalence settings (<20%). However, these areas may still have a prevalence of M&HI >2% in preSAC, SAC and WRA14. In addition, epidemiological data from the Tumikia study suggest that with lower prevalence, the prevalence of M&HI is relatively higher due to increasing aggregation of parasites as MDA coverage rises, likely due to a small proportion of persistent non-compliers to treatment. A revision of the 20% threshold downwards seems desirable.
WHO guidelines for moderate-prevalence settings suggest annual PC of young children, preSAC, SAC and WRA. This may be sufficient to reach the morbidity target for settings where coverage is sufficiently high (75%) in the absence of systematic non-compliance to treatment.
For high-prevalence settings (>50%), WHO guidelines suggest semiannual treatment. Here the morbidity target is less likely to be achieved following current guidelines, especially for hookworm and T. trichiura. As the main burden of hookworm infection lies in adults, the morbidity targets will only be reached when also treating adults as a whole5, not just WRA8. Control of T. trichiura will require community-wide treatment with albendazole3 or dual treatment with ivermectin and a benzimidazole3,5. If systematic non-access to treatment remains high, meeting the target may not be feasible8.
Reaching the milestone in 2030 will require community-wide coverage and/or targeting of WRA (especially for hookworm), with low systematic non-access/non-compliance to PC and little coverage heterogeneity within PC implementation units. Modelling suggests that the timeline for achieving the target is expected to be longer if there is re-importation of disease, e.g. by migration for areas with low or no treatment coverage16. Meeting the target may require coordination of national STH programmes at country borders due to human movement.
After stopping or scaling down treatment (which is an option in the current WHO guidelines, see Figure 1), infection levels are likely to bounce back within one to two years17. Thus, it may not be possible to decrease the number of required tablets as proposed as a new WHO target5,8. This is further complicated by population growth between now and 2030, which could necessitate a further increase in the number of treatments required for pre-SAC and SAC. See Figure 1 for an alternative decision-tree based on recent modelling.
Our analyses suggest that uptake of effective WASH is needed to sustain the gains made by PC in the longer term10. If EOT is not achieved and PC is stopped or scaled down in the absence of effective WASH, the probability of resurgence is very high5. In the absence of effective WASH interventions, the sustainability of the morbidity targets is undermined by human population movement unless PC is continued indefinitely16.
Child-targeted treatment for hookworm is cost-effective at reducing morbidity in children, even in high-transmission settings18,19. Community-wide treatment is predicted to be more cost-effective in the longer term with respect to the overall morbidity case-years prevented than child-targeted treatment, as the main hookworm disease burden lies in adults18,19. Annual co-administration of albendazole/mebendazole with ivermectin is predicted to be more cost-effective than semi-annual albendazole/mebendazole treatment for reducing the prevalence of heavy T. trichiura infections in SAC9. In general, achieving high coverage and good individual compliance in annual treatment rounds may be more cost-effective than treating twice a year with lower coverage.
Population movement can re-import infection into a geographical area that has previously reached morbidity control or EOT. Measures to mitigate this risk include aiming for evenly high coverage across implementation units and co-ordination of programmes across country borders16.
Systematic non-access and non-compliance to treatment in repeated rounds of MDA and predisposition to heavy infection will create a pool of individuals with high infection burden that can re-infect others8,10,20–24. Increasing access as well as coverage will be important for achieving the 2030 targets22,23.
Reducing frequency of treatment, as proposed in WHO guidelines25 and the new goal for 2030 of reducing the number of tablets required for treating STHs, in the absence of EOT and/or effective WASH measures (including both measures reducing contamination and measures reducing exposure to infection) can lead to rapid resurgence of infection prevalences to pre-treatment levels5,10,26–28. See Figure 1 for an alternative decision-tree based on recent model-based analyses.
The morbidity target is defined in terms of the prevalence of M&HI. However, infection intensity does not necessarily reflect morbidity accurately, as light infections can be associated with non-negligible morbidity and the severity of symptoms associated with M&HI is highly variable29. Furthermore, current diagnostic tools have poor sensitivity at low prevalence levels. Defining targets depending on the diagnostic used seems desirable in future policy formulation.
Transmission dynamic models with parameters estimated from cross-sectional and longitudinal epidemiological data show that technically EOT is feasible for STH in some settings. It is predicted that EOT can be achieved in low-transmission settings where A. lumbricoides or T. trichiura are the dominant parasites by annual treatment of SAC, assuming 80% effective coverage and random compliance at each round of treatment30. Where EOT is feasible, it may be more cost-effective than continuous morbidity control, provided no re-importation occurs19. In high transmission settings, community-wide treatment is predicted to be more effective (especially for hookworm) and more cost-effective.
Another new WHO goal for 2030 is control of strongyloidiasis. This requires ivermectin treatment, which would particularly benefit areas with high prevalence of T. trichiura. Currently, for policy assessments there is only epidemiological data on Strongyloides stercoralis31,32 but no model-based predictions. As for the other STH, models will provide useful insights for policy formulation.
Future work that the NTDmc can contribute in support of the design and achievement of the WHO 2030 goals will focus on: 1) an analysis of the value of different diagnostic methods and sampling strategies on M&E of STH morbidity targets and predicting the probability of EOT; 2) understanding the role of spatial heterogeneity in prevalence and coverage and human population movement on STH control programmes; 3) investigating the risk of emergence of drug resistance as well as whether and how monitoring of drug efficacy may help, 4) quantifying the link between infection intensity and morbidity; and 5) assessing the importance of different patterns of individual compliance to treatment to achieving the WHO targets as data becomes available from large-scale epidemiological studies and trials. Other proposed topics for future work include the impact of discontinuation of lymphatic filariasis programmes on STH, infection models encapsulating molecular epidemiology data of who infects whom, defining threshold values for when systematic non-access and non-compliance causes failure to achieve WHO targets, and development of transmission models for Strongyloides stercoralis.
No data are associated with this article.
NTD Modelling Consortium discussion group on soil-transmitted helminths:
Carolin Vegvari2, Federica Giardina1, Veronica Malizia1, Sake J. de Vlas1, Roy M. Anderson2, Luc E. Coffeng1
1. Department of Public Health, Erasmus Medical Center, Rotterdam, The Netherlands
2. London Centre for Neglected Tropical Disease Research, Department of Infectious Disease Epidemiology, Imperial College London, London, UK
Corresponding author: Luc E. Coffeng (l.coffeng@erasmusmc.nl)
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for the Open Letter provided in sufficient detail?
Yes
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Yes
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Soil-transmitted helminth and schistosomiasis epidemiology, global health policy, public health policy and implementation.
Is the rationale for the Open Letter provided in sufficient detail?
Yes
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Yes
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: infectious disease epidemiology, public health program implementation and global health policy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 04 Dec 19 |
||
|
Version 1 23 Oct 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)