Keywords
gambiense human African trypanosomiasis (gHAT), sleeping sickness, WHO goals, elimination of transmission, NTD Modelling Consortium, prediction
This article is included in the 2030 goals for neglected tropical diseases collection.
gambiense human African trypanosomiasis (gHAT), sleeping sickness, WHO goals, elimination of transmission, NTD Modelling Consortium, prediction
The views expressed in this article are those of the authors. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the World Health Organization. Publication in Gates Open Research does not imply endorsement by the Gates Foundation.
Gambiense human African trypanosomiasis (gHAT, sleeping sickness) is an infection caused by the parasite Trypanosoma brucei gambiense, spread through blood-meal feeding by tsetse in West and Central Africa. Disease symptoms caused by gHAT generally progress over multiple years. Stage 1 disease is defined as the time before the parasite crosses the blood-brain barrier, with symptoms such as headache and fever, whereas stage 2 involves neurological symptoms and typically death if left untreated. Fortunately, there are a variety of tools available to assist in the control of gHAT and these have been effective at reducing the burden of gHAT from 37,385 cases in 1998 to 953 cases in 20181. The primary intervention against gHAT is large-scale, test-confirm-and-treat strategies with diagnosis and confirmation performed by mobile teams in at-risk villages, followed by hospitalisation for treatment; 56% of detected cases in 2016 were diagnosed in this way2. The rest of cases are identified through passive surveillance (self-presentation) in fixed health facilities. There are additional options to reduce transmission by targeting the tsetse vector directly, which has been implemented successfully in several regions, but is currently a non-standard component of intervention strategies in most areas.
Previously, the World Health Organization (WHO) roadmap set a target of elimination as a public health problem (EPHP) for gHAT by 20202, which has been redefined as (a) having fewer than 2000 globally reported cases and (b) at least a 90% reduction in areas reporting >1 case per 10,000 people over a five-year period (2016–2020) compared to a 2000–2004 baseline (Table 1)3. It is complex to determine yet whether the second indicator will be met without a detailed analysis of global data; however, 1419 cases were reported in 2017 and 953 were reported in 2018, suggesting that the first indicator should have been not only met but greatly surpassed. The subsequent WHO goal is global elimination of transmission (EOT) by 2030 (Table 1)4. Achievement of EOT for gHAT would not only represent a huge stand-alone accomplishment but would place gHAT amongst the very select group of infections for which, through deliberate intervention, global EOT has already been achieved (smallpox and rinderpest) or may be met by 2030 (e.g. Guinea worm and polio).
Predictive, mechanistic modelling is a data-driven approach to explore the feasibility of reaching the WHO goals, taking into account the known biology of infection but also representing uncertainty in all processes. Recent mathematical modelling by the NTD Modelling Consortium and collaborators - including groups from the Institute for Disease Modeling, the Swiss Tropical and Public Health Institute, University of Warwick and Yale University - has provided quantitative perspectives on the challenges of reaching and the likelihood of achieving both the 2020 and 2030 WHO goals for gHAT. The following sections outline some of the key model findings that are of direct relevance to the 2030 EOT goal.
In the last two decades, the predominant strategy against gHAT was medical only, comprising active screening and passive surveillance followed by treatment. Current medical-based gHAT control strategies are working well in reducing incidence2 and modelling indicates they are also reducing underlying transmission5,6. Shortening time to detection and treatment of cases further reduces morbidity and subsequent onward transmission7. Modelling indicates that, in Uganda and South Sudan, passive surveillance reduced transmission by 30-50% during the 1990s and 2000s; strengthening these systems in gHAT endemic regions could therefore have great potential8. Staged gHAT case data (differentiating between stage 1 and stage 2 cases) can provide substantial information on the effectiveness of, and changes in, the passive surveillance system; for example, improvement in time to detection in former Bandundu province in the Democratic Republic of the Congo (DRC) is reflected in a greater proportion of stage 1 cases9.
Despite these successes, controls can be disrupted by conflict or other events; notably, the Ebola epidemic in West Africa resulted in temporary cessation of medial activities10. Furthermore, in higher endemicity settings or regions with little screening, the current medical-only interventions are predicted to be insufficient for achieving EOT by 2030 (e.g. in several health zones in Bandundu, DRC, EOT is predicted to be realised after 2050)11–13. In these settings - assuming scale up of vector control (VC) is feasible and the substantial (>80%) reduction in tsetse density14,15 can be reproduced widely - supplementing medical interventions with VC is predicted to be cost-effective at relatively low willingness-to-pay (WTP) thresholds in high-risk areas13, and to lead to EOT in much shorter timescales (1–6 years instead of >30 years in some settings)11,12.
The WHO 2030 goal for gHAT is EOT globally, with the key proposed indicator of achieving zero reported cases (Table 1). Other proposed indicators relate to sustaining coverage of passive surveillance.
In the long term, reaching EOT will lead to zero detected cases; however, the two objectives are not equivalent - it is possible either that zero case detections could occur without EOT or, conversely, that gHAT detections could be observed even after EOT.
EOT before zero reporting. Achieving EOT may not immediately lead to zero detected cases as there is often a long period between infection and detection (several years is typical16, although in extreme cases this could be decades17). As EOT is approached, the choice of confirmatory diagnostics becomes increasingly important as imperfect test specificity, even current algorithms with ~99.9% specificity, can lead to false positive cases. As we approach the 2030 goal, more rigorous methodologies (e.g. the laboratory-based trypanolysis test with 100% specificity18) should help to circumnavigate this problem.
Zero reporting but not EOT. Achieving zero detected cases does not mean that there is EOT for numerous reasons. The first possibility is that screening does not identify all remaining infections at peri-elimination. Only some of the population at risk is regularly screened; modelling5,6,9 suggests that some high-risk individuals (~20% of the population) may not attend active screenings and data show that not all settlements in high-risk areas are screened annually (around 50% of villages in a high-endemicity region of DRC were screened in any given year4,19). Coverage may improve with mini mobile teams (screening otherwise inaccessible villages) or door-to-door screening (likely increasing the number of high-risk people participating), but pockets of infection could still be missed. Furthermore, large areas of DRC (Figure 1), South Sudan and Central African Republic with potential transmission are not regularly screened due to regional conflicts. Secondly, even where there is a functional health system, there is a high probability of underreporting; models for Bandundu, DRC, suggest that only around 20% of gHAT cases that escape active detection are identified by passive detection (Model W in Rock et al.9), corresponding to ~63% of all infections being unreported.
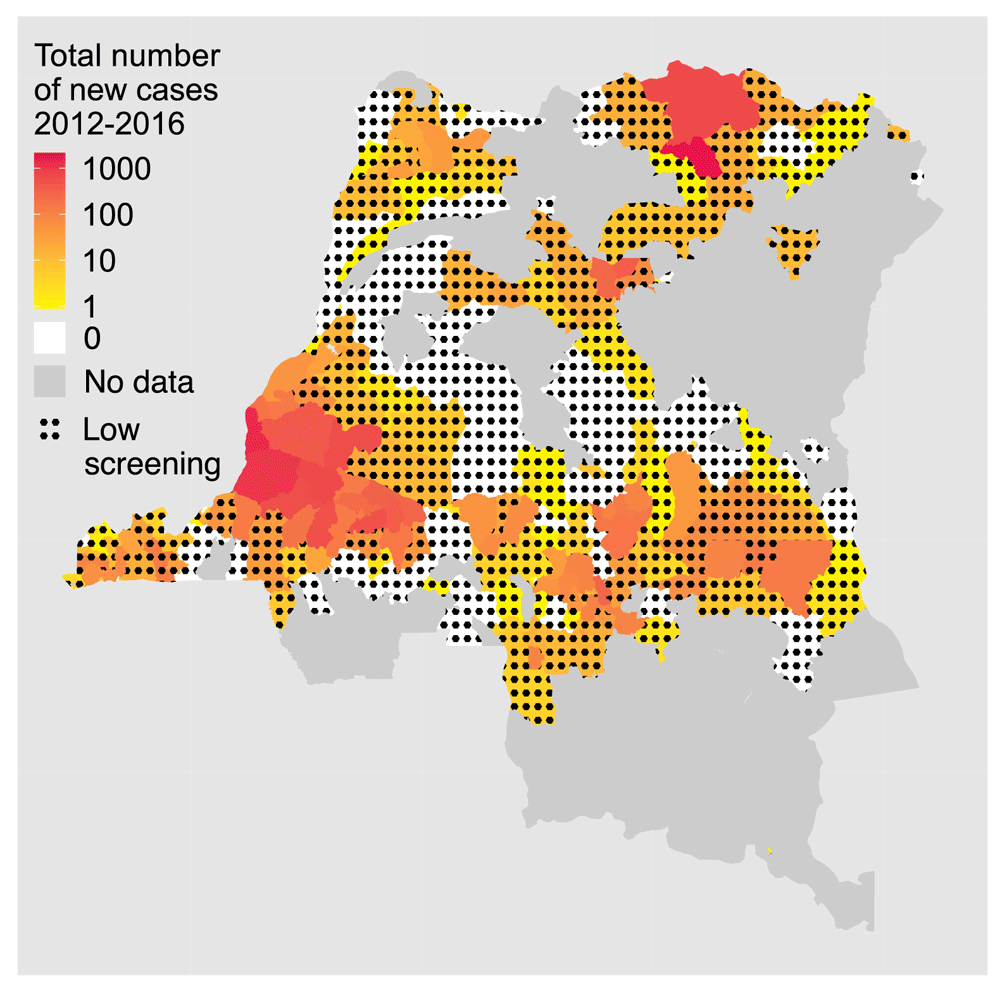
Colours represent numbers of reported cases in health zones from the last five years of data set. Health zones that never (2000–2016) report cases or active screening are coloured grey, whilst zones with <5% mean active screening coverage (during 2012–2016) are shown with black dots. This figure has been adapted from data presented in Franco et al.1 under a CC-BY 4.0 license and with permission from Dr Erick Mwamba Miaka, director of the National HAT Control Programme (PNLTHA) of the Democratic Republic of the Congo.
Models can be used in order to explore the predictive power of one or more years of zero detected cases in estimating the likelihood of EOT. A stochastic village-level model (replaying active screening from 2000-16 for 559 settlements in Yasa Bonga & Mosango, Bandundu, DRC) focussed on detecting zero cases under active screening. This model strongly indicates that three or more consecutive rounds of finding zero cases is sufficient to reach >90% positive predictive value (PPV) of local EOT across typical village sizes and where screenings that achieve <10% coverage were ignored (Figure 2)19. There is higher certainty of EOT in smaller settlements and only using active screenings with >50% coverage as a measure could reduce the number of screening rounds needed to have high confidence. Current WHO guidelines recommend conducting three consecutive years of active screening with zero detections in a village before stopping7, therefore providing high confidence of local EOT prior to cessation. Modelling suggests that factoring screening coverage and population size into future guidelines could further improve certainty that EOT is met before stopping and could reduce the number of zero detections required for smaller settlements if coverage is sufficient. Scaling these insights from the village to larger spatial scales is confounded by spatial correlations and reinvasion, suggesting an intelligent and reactive surveillance methodology is required.
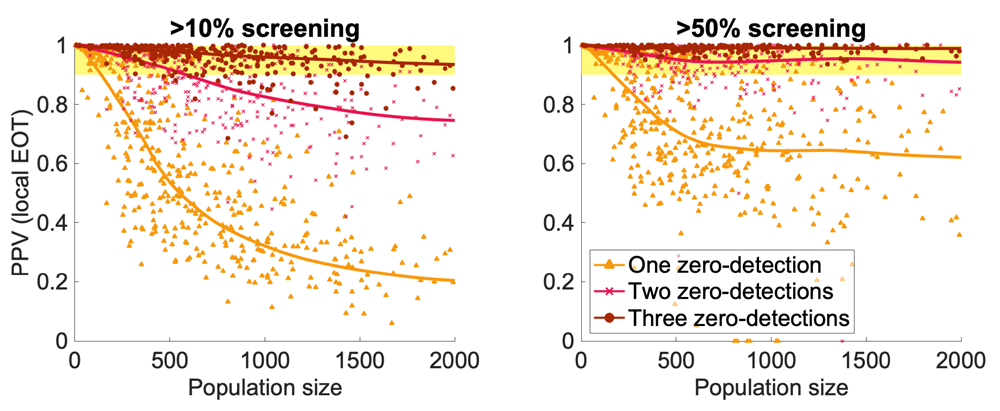
The positive predictive value (PPV) of zero case detections to assess whether local EOT has occurred is the probability of zero human infection given consecutive active screenings with no cases found and no passive reporting in between (for >2 screenings). Model parameterization is for Yasa Bonga and Mosango health zones in the Democratic Republic of the Congo. The left figure uses all active screenings with >10% coverage, while the right figure is restricted to screenings of >50% coverage. The yellow region indicates >90% confidence that EOT has been met locally. This figure has been reproduced with permission from Davis et al.19.
Finally, there is potential for circulation of infection in animal reservoirs or persistence in asymptomatic individuals, which could lead to resurgence even after zero human reporting20.
Models predict that for some regions (e.g. Equateur, DRC) continuation of the current medical-only strategy could achieve local EOT by 203011; however, in other regions (particularly some of Bandundu, DRC) this strategy may need to be supplemented with additional or improved interventions, even in areas likely to meet EPHP by 202012. Local EOT may be unsustainable without continued control due to the risk of reinvasion from other infected areas.
Multiple modelling approaches have shown that improvements to passive surveillance, targeting active screening to include high-risk groups, and VC could all result in reduced transmission and lead to EOT by 2030 with higher probability than the current medical-only strategy9,21. Whilst it may not be necessary to implement VC across all settings, modelling consistently finds that VC averts infections fastest amongst the considered strategies. Modelling also suggests that the use of other new technologies (i.e. new oral drugs and RDTs) could lead to EOT but with lower probability and slower timelines than VC13.
Modelling results are generally based on assumptions that the health system retains similar or better functionality over the next 10 years. Political instability, conflict, or a reduced priority for tackling the reduced number of future cases could all lead to less control being applied in the future. Models are therefore making assumptions that screening and other controls follow recent trends.
Stopping large-scale control activities against gHAT too soon could be problematic for EOT. Modelling was used to explore potential resurgence following attainment of EPHP in Guinea22, concluding that the presence of animal reservoirs would likely lead to resurgence following cessation of screening and vector control, but resurgence was unlikely if transmission was anthroponotic. Indeed, interruption of medical interventions in Guinea during the Ebola outbreak has shown that early cessation of activities in low prevalence settings can still lead to resurgence over three years10. In contrast, regions that maintained VC but stopped medical intervention observed a decrease in prevalence during the same time period23.
Even if an area has reached local EOT, there is a concern that cessation of activities could be risky if nearby places have on-going transmission. Modelling reinvasion of HAT in villages in DRC that have achieved local EOT suggests short-term reinvasion is likely (>70%) from a single infected person, but less likely to cause persistent infection for ~15 years (<20%)19.
The burden of NTDs falls in resource-poor settings, and it is of utmost importance to efficiently use the resources available. A cost-effectiveness analysis for gHAT across settings of different transmission intensities has found that VC combined with other new technologies (diagnostics and drugs) is likely to be highly cost-effective in high-transmission settings (i.e. cost-effective for WTP thresholds >$386/disability-adjusted life year [DALY] averted). In moderate-transmission settings this strategy is only likely to be cost-effective for high WTP thresholds (>$1509/DALY averted), with medical-only strategies using new technologies likely to be preferable for lower WTP thresholds13. Unfortunately, cost-effectiveness in this traditional net-benefits framework does not always align with the goal of EOT by 2030, as strategies that are cost-effective (in terms of DALYs versus costs) may not be sufficient to meet the EOT goal. For example, in Sutherland et al.13, VC strategies were generally required to have high predicted probability of EOT by 2030, despite having low probabilities of being cost-effective in moderate- or low-transmission settings.
An analysis on the affordability of gHAT intervention and patient financial impact estimated that the total costs of a global control or elimination programme would be substantial (depending on the programme, between US$410.9 million and US$1.2 billion, compared to US$630.6 million for control activities in 2013-2020)24. Alleviation of impoverishment and catastrophic health expenditures for households due to gHAT infection can only be achieved through elimination, rather than control, programmes.
A more complete review of key factors that may impact the EOT goal is given by Büscher et al.20. Here, insights arising from modelling-based studies are discussed.
Several modelling studies suggest that there is systematic non-participation in active screening, with high-risk individuals less likely to participate; the models without this heterogeneity were unable to match the observed longitudinal patterns of cases across different regions5,6. More detailed data on age and gender of screening participants and gHAT cases could help better elucidate key groups in the population most responsible for transmission.
Although prevalence of gHAT infection in animals is nonzero, estimates are uncertain and the role of infected animals in onward transmission is unclear20. One modelling study using point prevalence data from animals in Cameroon suggested that animals constitute a possible transmission reservoir, implying that control targeting only human cases would be unable to eliminate gHAT due to persistence in animals25, whereas modelling using longitudinal human data (Guinea, DRC, Chad) suggest that there is comparable statistical support for models with and without an animal reservoir5,6,22. However, animals are unlikely to be able to sustain transmission on their own in Chad5.
Modelling suggests that VC, including spraying livestock, would reduce any possible transmission from animals, although pockets of sustained transmission could occur away from human activities26.
Asymptomatic infections in humans have been considered in a few transmission models. In some9,21, the role of these infections in maintaining transmission or causing resurgence was unclear, although one modelling study using data from Guinea found both asymptomatic and clinical human infections were necessary for gHAT to persist (assuming no animal reservoir) and concluded that passive surveillance alone was not sufficient for gHAT monitoring in the approach to elimination27.
So far, little attention has been paid to the movement of people in the modelling literature on gHAT; however, this may be important in areas that recently achieved disease-free status. Particular regions of concern would include formally endemic areas with both high influxes of refugees/internally displaced people and limited surveillance.
Table 2 highlights a list of priority questions for modellers that are of relevance for the 2030 EOT goal for gHAT arising from discussions between modellers and WHO.
No data are associated with this article.
NTD Modelling Consortium discussion group on gambiense human African trypanosomiasis (gHAT):
Kat S Rock (k.s.rock@warwick.ac.uk; corresponding author)1,2, Ronald E Crump (r.e.crump@warwick.ac.uk)1,2, Christopher Davis (c.davis.3@warwick.ac.uk)1,2, María Soledad Castaño (soledad.castano@unibas.ch)3,4, Marina Antillon (marina.antillon@swisstph.ch)3,4, Ching-I Huang (ching-i.huang@warwick.ac.uk)1,2, Maryam Aliee (maryam.aliee@warwick.ac.uk)1,2, Fabrizio Tediosi (fabrizio.tediosi@swisstph.ch)3,4, Nakul Chitnis (nakul.chitnis@unibas.ch)3,4, Matt J Keeling (m.j.keeling@warwick.ac.uk)1,2,5
1Mathematics Institute, University of Warwick, Coventry, CV4 7AL, UK
2Zeeman Institute for Systems Biology and Infectious Disease Epidemiology Research (SBIDER), University of Warwick, Coventry, CV4 7AL, United Kingdom
3Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Socinstrasse 57, Basel, 4051, Switzerland
4University of Basel, Peterplatz 1, Basel, 4051, Switzerland
5School of Life Sciences, University of Warwick, Coventry, CV4 7AL, UK
We would like to thank Joshua Longbottom, Benjamin Amoah and Michelle Stanton for contributing to the priority questions in this article. We thank members of the Gates Foundation NTD team and WHO NTD team for providing valuable feedback on this article. Additionally, we are grateful to Andreia Vasconcelos for overlooking the development of this article. We thank Dr Erick Mwamba Miaka for his permission to include the DRC data map (Figure 1).
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for the Open Letter provided in sufficient detail?
Yes
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Yes
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Yes
Competing Interests: No competing interests were disclosed.
Is the rationale for the Open Letter provided in sufficient detail?
Yes
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Yes
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: African trypanosomiasis, drugs mode of action and resistance mechanisms
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 21 Apr 20 |
read | |
|
Version 1 07 Oct 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)