Keywords
Chagas disease, WHO guidelines, Elimination as a public health problem, intradomiciliary transmission interruption, trypanocidal treatment, NTD Modelling Consortium
This article is included in the 2030 goals for neglected tropical diseases collection.
Chagas disease, WHO guidelines, Elimination as a public health problem, intradomiciliary transmission interruption, trypanocidal treatment, NTD Modelling Consortium
BNZ, benznidazole; BTT, blood transfusion transmission; CD, Chagas disease; CTI, congenital transmission interruption; EMTCT Plus, elimination of mother-to-child transmission of HIV, syphilis, Chagas, and perinatal hepatitis B; EPHP, elimination as a public health problem; FOI, force-of-Infection; IDTI, intradomiciliary transmission interruption; IRS, indoor residual spraying; NFX, nifurtimox; NTD, neglected tropical disease; PAHO, Pan American Health Organization; PCR, polymerase chain reaction; PPC, proportion of parasitological cure; qPCR, quantitative PCR; R&D, research and development; TTT, tissue transplant transmission; WHO, World Health Organization; WISCC, World Information System for the Control of Chagas Disease; 95% CI, ninety-five percent confidence interval.
The views and opinions expressed in this article are those of the authors and do not necessarily reflect those of the World Health Organization. Publication in Gates Open Research does not imply endorsement by the Gates Foundation.
With an estimated 8–10 million cases worldwide, Chagas disease (CD; also known as American trypanosomiasis) remains a major cause of heart disease morbidity, mortality and economic burden, particularly in endemic Latin American countries1. CD is a parasitic disease caused by the protozoan Trypanosoma cruzi, and transmitted mainly by domiciliated triatomine (Reduviidae) vectors (kissing bugs) in tropical areas of the Americas. However, along with the urbanization process in recent decades, other transmission routes, such as blood transfusion, organ transplant and congenital have become important in both endemic and non-endemic countries2. The disease is characterized by a long asymptomatic period (years to decades) before reaching the full set of clinical manifestations that include heart failure, arrythmias, stroke, digestive complications and other conditions that increase the risk of death3.
From the beginning of the Southern Cone Initiative in the 1990s, most endemic countries have made great progress towards the control of T. cruzi transmission by implementing mainly vector control (chiefly through indoor residual-insecticide spraying (IRS)) and blood transfusion control via donor screening. So far, 11 out of the 21 recognised endemic countries have been certified as having reached intradomiciliary transmission interruption (IDTI)4. However, the real impact of such interventions has not been rigorously documented and quantified, and various concerns have been raised around the relationship between reaching the various operational thresholds that have been proposed and truly achieving interruption/elimination of transmission and reduction in morbidity5. Also, the increased health-care demands from the chronically-affected populations, and the limited offer of diagnosis and trypanocidal and supportive treatment pose additional challenges to CD control.
The World Health Organization (WHO) has set goals for the control of CD by 2030 in both endemic and non-endemic countries, including achieving the target of elimination as a public health problem (EPHP), the interruption of the various transmission routes and the scale-up of diagnosis and treatment strategies (a summary of these goals is presented in Table 1). In order to help evaluate progress towards and feasibility of these goals, mathematical modellers from different countries have joined forces, under the invitation of the Bill & Melinda Gates Foundation-funded NTD Modelling Consortium, to contribute to a joint analysis of mathematical modelling insights to support the CD WHO goals for 2030. In February 2018 a workshop on “How can modelling contribute to achieving the goals for Chagas disease in the Horizon 2020 and beyond?” was held at Imperial College London, with participants contributing ideas on how to quantitatively inform global progress on control and elimination of CD. In this document, we consolidate the main points from these discussions, involving ecological and epidemiological modellers and researchers from Imperial College London and Sussex University UK, Princeton University and University of Pennsylvania USA, University of Perpignan Via Domitia France, University of La Plata and CONICET Argentina, and Fundação Oswaldo Cruz – Fiocruz, Brazil.
A main planned target is achieving IDTI in endemic countries with 0% colonization of dwellings and 0% incidence of T. cruzi-infected persons (Table 1). The accumulated body of knowledge from the modelling work that has been undertaken by these groups over the last years tends to agree on various aspects, particularly the important progress made towards IDTI. Reductions of incidence and disease burden through the control or elimination of introduced/non-native Triatoma infestans (in some areas of the Southern Cone such as Brazil, Uruguay, and pockets of Paraguay, Chile, Peru, and Argentina) and of Rhodnius prolixus (in Central America and some pockets in Northern South America) have been achieved by a combination of IRS, locally practicable environmental management strategies and housing improvement, as initially suggested by pioneer mathematical modelling6. However, enormous challenges and limitations persist in terms of sustainability, data availability to monitor progress or re-emergence (which includes vector re-introduction (or resurgence from residual foci) and re-emergence of transmission), and clarity in the specific strategies to be undertaken towards the achievement of the 2030 goals.
IDTI is potentially achievable in epidemiological settings with exclusively domiciliary (i.e., non-native) vectors and no insecticide resistance. Pioneer work also illustrated an application of triatomine-population modelling to optimize IRS for vector control, suggesting how to determine the optimal timing of spraying for the control of T. infestans in Argentina, depending on the season and the structure of the triatomine population7. Using a similar approach with current techniques will be useful to help programmes to better target IRS strategies against domiciliated vectors. The critical (threshold) number of triatomines per house related to transmission risk, and how this can be used to prioritize vector control campaigns, has been investigated. A relationship between house infestation (proportion of houses infested) and the number of triatomines/house was fitted to data from various locations prior to vector control and applied to cases where there was only one triatomine species present in the dwellings, as well as to situations with mixtures of species and developmental stages, various types of houses and bug densities per house. These relationships can be improved when data are stratified (if available) with some critical co-variates, such as house-construction materials, number of humans and zoonotic hosts in the dwellings, and IRS status8,9. Understanding further these relationships taking into account other variables (such as the distribution of triatomines per house, the triatomine species, the time elapsed after intervention, etc.), will be crucial to determine operational thresholds that lead to cost optimisation10.
However, large areas of endemic countries with domiciliated vector species have sylvatic populations (for which traditional vector control measures are less effective). An example is Rhodnius prolixus, a vector that has been targeted for elimination in Central and South America. Unlike Central America, where R. prolixus is strictly domiciliated11, large areas of Colombia and Venezuela have R. prolixus as the most prominent sylvatic species. In these areas re-colonisation occurs readily between 1 to 67 months after IRS12. In the presence of sylvatic populations, there is a continuous introduction and colonisation of domiciliary and peri-domiciliary habitats; in these areas, traditional vector control is not feasible in a sustainable manner. Additionally, even if the domiciliated vector species are eliminated, their niche could be taken over by other sylvatic vector species.
Various studies on routine vector surveillance have demonstrated that the currently used methods have low sensitivity and greatly underestimate vector density, infestation and infection rates; vector surveillance may be capturing half of infestations – and, most likely, most bugs within a house13,14. These shortcomings may have greater impact in low-prevalence and post-intervention settings.
Measuring 0% incidence requires analysis of seroprevalence studies, but diagnostic tests do not have perfect sensitivity and specificity. Investment in Research and Development (R&D) is essential to improve the performance of serology-based tests, particularly in near-elimination (low-prevalence) settings. Also, hierarchical models can be used to estimate test performance parameters (sensitivity and specificity) and then correct infection frequency14. The most informative age classes for seromonitoring should be identified, and strategies developed for monitoring the long-term response to control. Modelling the historical force-of-infection (FOI; the per susceptible incidence rate) using population representative seroprevalence studies, is a promising quantitative tool to measure trends in incidence and achievement of operational thresholds for transmission interruption as done in Peru and Colombia15,16 (e.g. <2% seroprevalence in under 5-year-olds) (Figure 1). However, even if IDTI were achieved, the likely presence of remaining vector populations, the protracted temporal scale of T. cruzi transmission (decades), and the long asymptomatic period of infection, can lead to many years passing before parasite re-emergence is noticed. FOI (catalytic) models are also a promising tool to estimate time to resurgence when a strategy has not been sufficiently effective, as applied in La Joya, Peru15 and the Bolivian Chaco17.
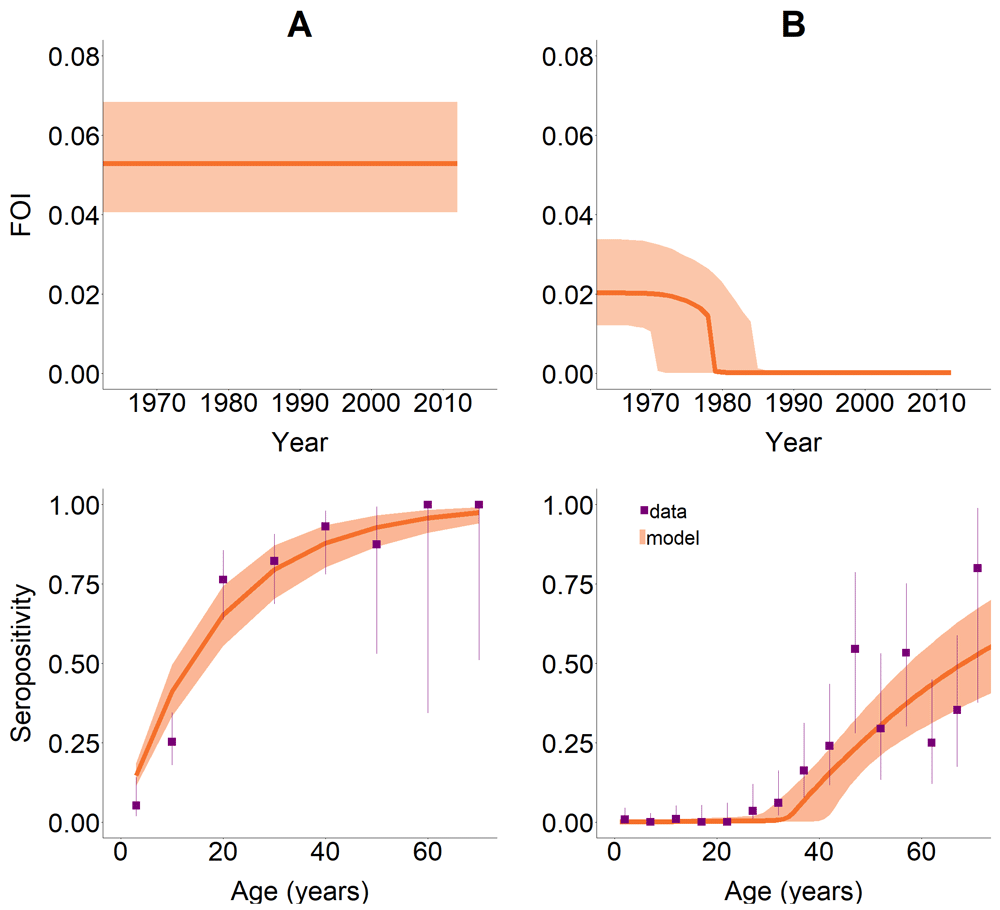
Upper panels represent the (modelled) historical FOI. Lower panels present the data (solid squares) and the modelled seroprevalence (orange shaded area) in: (A) a non-intervened area with a long-established endemic situation and (B) a successfully intervened area. This figure has been reproduced from 15 under a Creative Common Attribution 4.0 International Licence (CC BY 4.0).
Modelling studies have indicated that potentially combining highly effective vector control with trypanocidal treatment of humans residing in endemic areas would substantially reduce the time required to achieve operational serological thresholds for IDTI as well as infection incidence and prevalence18 (Figure 2). Understanding the implications of this combination of interventions for achieving elimination of transmission and EPHP needs further work.
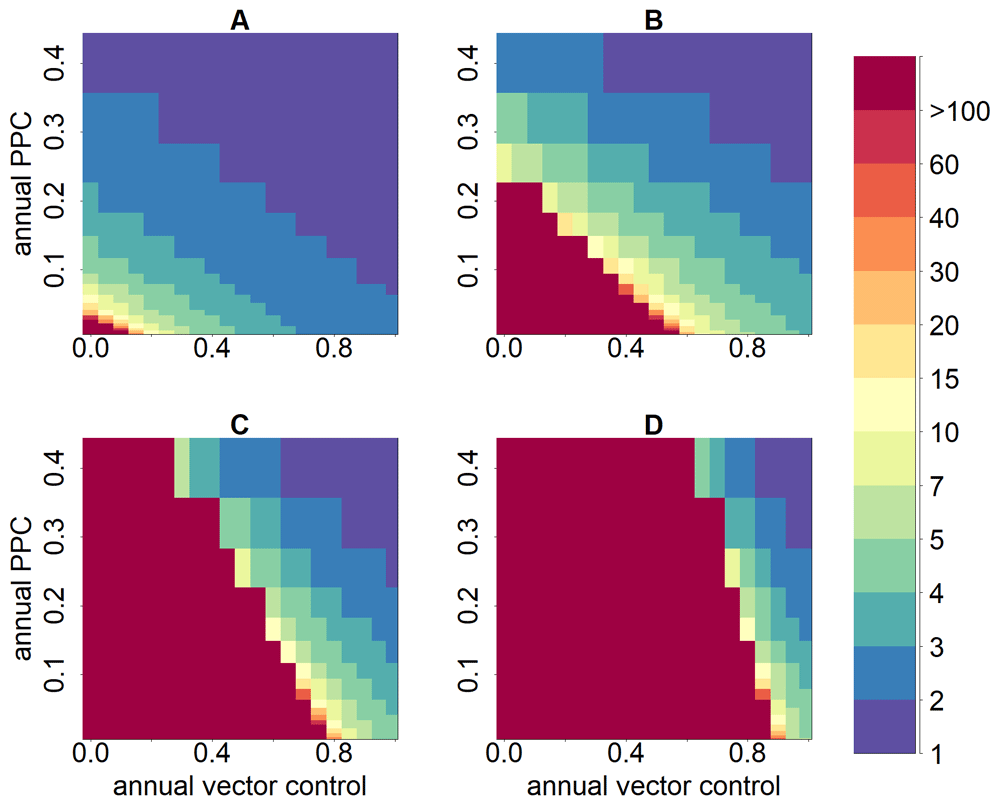
Annual vector control defines the proportion by which vector density is annually reduced (0–100%); parasite clearance is measured as proportion of parasitological cure (PPC); annual PPC defines the proportion of humans effectively treated annually with trypanocidal treatment, i.e., the percentage of the infected human population achieving parasitological cure (0–40%). The impact of the combined strategies is measured on the number of years necessary to reduce seroprevalence in children aged <5 years to <2% (the operational serological criterion for intradomiciliary transmission interruption), as represented by the colour scale. The panels represent: (A) low; (B) moderate; (C) high; and (D) very high endemicity levels. This figure has been reproduced from 17 under a Creative Common Attribution 4.0 International Licence (CC BY 4.0).
Native triatomines maintain extensive enzootic T. cruzi transmission cycles from the United States of America to Patagonia, including vast areas where the three main domestic vectors of human CD (Triatoma infestans, T. dimidiata, and Rhodnius prolixus) occur in the wild, with the potential for invading, infesting, and re-infesting dwellings. Challenges remain concerning the role and magnitude of rural and urban transmission, which are harder to quantify. In vast areas of endemic countries, intradomicilary transmission is due to ‘sylvatic’ species that do not colonize domiciles but only make occasional visits (also called ‘intrusive’ species)19. This has led to researchers to suggest an alternative classification of triatomines that captures their complexity but is still operationally relevant for surveillance20.
Recent studies have modelled the relative roles of some candidate variables on house invasion by sylvatic triatomines21. Modelling studies suggest that a better comprehension of vectorial transmission in rural and urban settings would require understanding and quantifying of two different forms of vector dispersal, namely, dispersal between sylvatic and non-sylvatic habitats and diffusive dispersal within cities22.
A promising avenue is the application of orthogonal polynomials methods to predict triatomine dispersal based upon exclusively life-history data of each triatomine species23. Tackling transmission by native vectors may necessitate alternative control strategies, which would require measuring dispersal and evaluating the efficacy of strategies such as those used for other vector-borne diseases, e.g. (impregnated) nets. Examples of the application of transmission dynamics and statistical models to evaluate both dispersal and the potential efficacy of such alternative strategies in comparison to typical IRS have been discussed22.
Another main target planned is achieving 75% access to trypanocidal treatment in T. cruzi-infected people with medical indications (Table 1). Trypanocidal treatment with benznidazole (BNZ) or nifurtimox (NFX) has been aimed at both reducing parasitaemia and curbing disease progression. So far, there is limited evidence on the efficacy of drugs for these.
Monotherapy with BNZ has been proven to reduce parasitaemia in up to 86.7% of treated patients24. However, it is known that the trypanocidal effect of BNZ varies across regions25,26. The evidence of trypanocidal efficacy for NFX is more scarce than for BNZ; unlike BZN, there is only one completed trial with 27 people treated and 24 placebo controls27. According to a 2014 Cochrane systematic review, there is not robust evidence yet regarding efficacy on halting or delaying clinical progression28. Before the BENEFIT (BENznidazole Evaluation For Interrupting Trypanosomiasis) trial (a randomized trial of BNZ for chronic Chagas’ cardiomyopathy)26, observational studies had indicated a possible impact on disease progression and mortality. However, the BENEFIT trial was not able to demonstrate such effect29. Criticisms about the design and very optimistic assumptions about the true effect of trypanocidal treatment have been raised about this trial. BENEFIT’s authors designed this trial assuming a high (26%) reduction relative to placebo in the incidence of cardiac complications among individuals with moderate to advanced cardiac disease. The trial identified a small (7%), but apparently consistent reduction of such outcomes, which may still be relevant for patients and from a public health perspective. For that small, but still relevant effect size, its sample size may have been underestimated (low-quality evidence for imprecision and lack of consistency with other studies, following GRADE criteria)30. Using the current regimens of BNZ or NFX (60-day treatment course), only 70% of patients adhere to treatment on average, mostly due to adverse effects26. However, alternative regimes with shorter duration or lower doses have been trialled (e.g. BENDITA trial31) with promising results. This could lead to crucially improved adherence. Finally, an earlier diagnosis of cardiomyopathy and more comprehensive (supportive) treatment for heart failure may reduce mortality and hospitalizations by 20–30% (assuming, and yet to be tested, that such treatment has an effect similar to other causes of cardiovascular disease).
Modelling work has shown that an optimal combination of parameters such as: coverage of screening; performance of diagnostic tests; proportion of people treated; and efficacy of trypanocidal drugs is crucial to the scale-up of diagnosis and treatment programmes. While screening and access to treatment can be incremented as part of strengthening health systems, improving diagnostics performance and drug efficacy will require concerted efforts18. With the current tools, low access to screening is the bottleneck; achieving just 10% of successful treatment at population level will require an enormous investment on improving access to screening, especially when targeting asymptomatic populations in low prevalence settings, which currently prevail in most endemic areas18 (Figure 3).
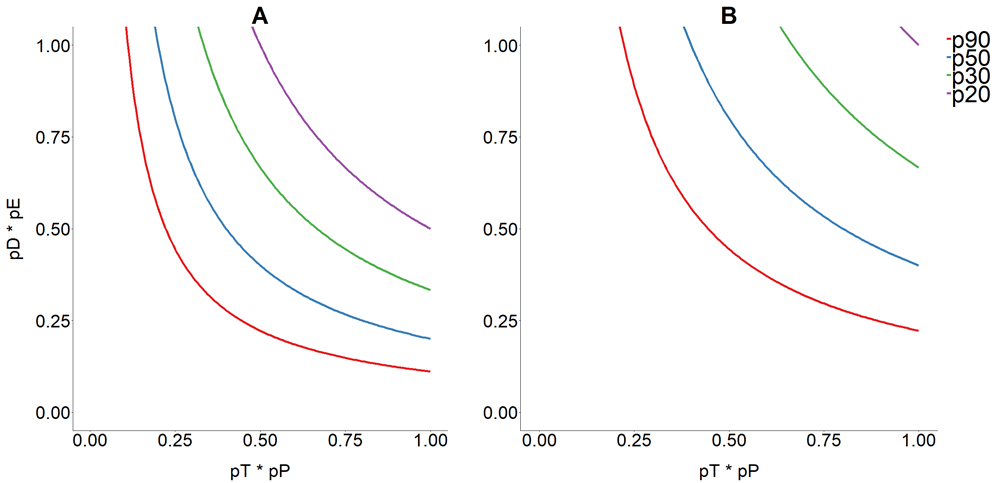
(A) 10% proportion of parasitological cure (PPC); and (B) 20% of PPC in a Trypanosoma cruzi–infected human population based on the combined probability of being diagnosed and treated (with trypanocidal medication) for Chagas disease. The horizontal axis represents the combined contribution of diagnosis as a product of the proportion of infected people who are tested (pT) and the proportion of those tested with a positive test result, that is, the sensitivity of the test (pP). The vertical axis represents the combined contribution of treatment, as the product of the proportion of those testing positives who are treated with currently available trypanocidal drugs (pD) and respond to treatment by clearing parasites according to efficacy (pE). Coloured lines represent the proportion (p) of infected people who would have to be reached by a test-and-treat programme (90% [blue], 50% [red], 33% [green], and 20% [orange]) to achieve the desired level of effective PPC. This figure has been reproduced from 17 under a Creative Common Attribution 4.0 International Licence (CC BY 4.0).
Achieving IDTI will require enhanced detection methods for domiciliated triatomines in low-infestation and low-prevalence settings. In order to monitor progress, improving the design of serological surveys for low-endemicity settings will be critical. FOI modelling suggests that increasing the age range for seromonitoring, instead of limiting it to under 5s or under 15s (e.g. using all-age classes), would be useful to understand temporal changes in T. cruzi incidence and the impact of interventions. Also, recent modelling studies have tested strategies to integrate data and models to guide interventions in Arequipa, Peru32, which can be used to improve cost-effectiveness. Models of triatomine dispersal and colonization, with evidence-based parameterisation, are also needed to both understand their dynamics and design and test alternative control strategies22.
Current estimates of access to diagnostics and treatment are at <1%33. Reaching 75% by 2030 does not seem feasible using the current passive surveillance system. This goal would only be feasible if an optimized screening/treatment strategy is purposely designed for the targeted countries. Scaling-up current strategies needs a substantial commitment by such countries, and resource availability will be an issue. Also, the availability of current drugs is suboptimal, and there is a recurring need for supportive medical treatment of the CD sequelae.
In order to achieve 100% coverage of screening strategies implemented in blood banks and transplantation centres, a surveillance and administrative system needs to be put in place. Specifically, this requires more inter-sectorial collaboration, the participation of insurance companies, private/public institutions, the implementation of clear protocols, and a substantial commitment of health systems to ensure full documentation of the process. The WHO has a project in progress to develop an exhaustive database called the “World Information System for the Control of Chagas Disease” (WISCC), with an agreement with the Computer Centre of the Polytechnic University of Barcelona (Spain), that may be functional to these needs. Additionally, modelling could inform how widely screening should be done in non-endemic countries.
Achieving most of the goals currently stated for women and newborns seems challenging with the currently available tools (Table 1). Given the long asymptomatic period and the current passive surveillance system for identifying cases of CD in endemic and non-endemic countries, it seems unfeasible that 90% of women of childbearing age will be screened. However, reaching almost 100% of pregnant women is potentially feasible with the recent strategy EMTCT plus (elimination of mother-to-child transmission of HIV, syphilis, Chagas, and perinatal hepatitis B), which adds mandatory surveillance tests during pregnancy for CD, planned to be in place in Colombia, Chile and Uruguay as pilot countries over the next few years34. However, it is currently unrealistic to achieve 100% of treatment in newborns infected, as the sensitivity of micro-haematocrit methods (with repeated test) has been estimated at 34.2%35. Repeated PCR-based tests can improve sensitivity up to 84.2%, but these tests are neither standardised nor widely available in endemic settings35. Controlling congenital Chagas transmission would require urgent research for new diagnostics and drugs/drug regimes. Increased medical training and availability of tests and drugs would also need to be markedly improved. Higher sensitivity to detect congenital cases could also be achieved by including not only newborns, but also infants, for whom serological testing can be used from 8 months of age36. Finally, careful planning and organization would also be essential for reaching and covering the more inaccessible rural (and indigenous) populations, which are likely to contribute disproportionately to the burden of CD.
Certifying intradomiciliary transmission interruption (IDTI) when such transmission has not truly been eliminated is the biggest risk. Current diagnostic tools are unlikely to be able to determine when true elimination has been achieved. However, it is perceived by public health officials that not having some reward system may harm even further the willingness of the countries towards elimination efforts. As countries reach low incidence, they may feel that efforts can be slackened. Determining the risk of re-colonisation after vector control is stopped and not having the tools to identify resurgence in a timely manner will, therefore, be important challenges. Developing and validating tools to quantify these risks will, in turn, inform ongoing initiatives to refine the process of re-certification that would follow after a (so far unspecified) number of years of the initial certification.
In terms of scaling-up treatment, it is anticipated that rare adverse effects of currently available drugs will become more evident. Also, in low-endemicity settings, the absolute number of false positives will be substantial as the number of people tested increase, even if specificity is high (98%, as estimated in a recent meta-analysis)37. Similarly, treating false-positive cases can become a particularly important issue, as the absolute number of such cases will increase when diagnosis implementation and access increases.
Currently, endemic and non-endemic countries have been working on their own according to their priorities and resources, but a transition to realistic elimination goals at a global scale will require the concourse of both governmental and non-governmental organizations.
IDTI is only feasible in a few areas of exclusively domiciliated, non-native vectors. Once IDTI is achieved in a region, and the control programme is stopped, surveillance will be needed to detect resurgence. Currently, this occurs only over a few years after initial certification, but there is little knowledge and guidance available for this post-certification surveillance.
Large areas in endemic countries are populated by sylvatic triatomine species for which traditional vector control is not effective in a sustainable manner. For those areas more experimental and modelling work is needed to better understand both transmission and control strategies.
Scaling-up diagnosis and treatment strategies will require not only a greater commitment of the health systems but also an important investment in terms of R&D for diagnostics and treatment strategies.
Table 2 outlines the priority modelling questions for further research that were elaborated in discussion with the WHO.
No data are associated with this article.
Collaborating Group on Chagas Disease Modelling:
Zulma M Cucunubá (zulma.cucunuba@imperial.ac.uk, corresponding author)1,2; Pierre Nouvellet (pierre.nouvellet@sussex.ac.uk)3; Sébastien Gourbière (gourbiere@univ-perp.fr)4; Juan-Carlos Villar (jvillarc@cardioinfantil.org)5,6; Jorge E Rabinovich (jorge.rabinovich@gmail.com)7; Michael Z Levy (mzlevy@pennmedicine.upenn.edu)8; Fernando Abad-Franch (abadfr@yahoo.com)9,10; Andy P Dobson (dobson@princeton.edu)11; Maria-Gloria Basañez (m.basanez@imperial.ac.uk, corresponding author)1,2
1 MRC Centre for Global Infectious Disease Analysis (MRC-GIDA), Department of Infectious Disease Epidemiology, School of Public Health, Imperial College London, London W2 1PG, UK
2 London Centre for Neglected Tropical Disease Research, Department of Infectious Disease Epidemiology, School of Public Health, Imperial College London, Norfolk Place, London W2 1PG, UK
3 Infectious Diseases Modelling Group, University of Sussex, Sussex House, Brighton BN1 9RH, UK
4 UMR 5096 'Laboratoire Génome et Développement des Plantes', Université de Perpignan Via Domitia, Perpignan, France
5 Departamento de Investigaciones, Fundación Cardioinfantil. Instituto de Cardiología, Bogotá, Colombia
6 Grupo de Cardiología Preventiva, Facultad de Ciencias de la Salud, Universidad Autónoma de Bucaramanga, Bucaramanga, Colombia.
7 Centro de Estudios Parasitológicos y de Vectores (CEPAVE, CCT La Plata; CONICET, Universidad Nacional de La Plata), La Plata, Provincia de Buenos Aires, Argentina
8 Department of Biostatistics, Epidemiology and Bioinformatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA
9 Grupo Triatomíneos, Instituto René Rachou, Fundação Oswaldo Cruz - Fiocruz, Belo Horizonte, Minas Gerais, Brazil
10 Núcleo de Medicina Tropical, Universidade de Brasília, Brasília, Distrito Federal, Brazil
11 Department of Ecology and Evolutionary Biology, Princeton University, New Jersey, USA
We would like to thank the participants of the “Imperial College Chagas Symposium”, 14–15 February 2018, for valuable insights on the ideas discussed in this article: María Dolores Bargues (University of Valencia); Luis Gerardo Castellanos (Pan American Health Organization); Lorenzo Cattarino, Julia Halder and Elisa Sicuri (Imperial College London); Orin Courtenay and Raquel Goncalves (University of Warwick); T Déirdre Hollingsworth (University of Oxford) and Mauricio J Vera (Ministry of Health, Colombia); the Infectious Diseases Data Observatory (IDDO); Isabela Ribeiro and Sergio Sosa-Estani (DNDi); and the Ministry of Health, Brazil. We also acknowledge David E Gorla (CONICET, Argentina) for his contributions during the World Health Organization meeting “Achieving NTD Control, Elimination and Eradication Targets Post-2020: Modelling Perspectives and Priorities”, Geneva 15–16 April 2019.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for the Open Letter provided in sufficient detail?
No
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Partly
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Yes
References
1. Gürtler RE, Kitron U, Cecere MC, Segura EL, et al.: Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina.Proc Natl Acad Sci U S A. 2007; 104 (41): 16194-9 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: JEC: Mathematical population biology and ecology, including mathematical modelling of infectious diseases, especially Chagas disease HzD: Quantitative ecology, population genetics, disease modelling REG: Ecology, epidemiology and control of vector-borne pathogens, especially Chagas disease.
Is the rationale for the Open Letter provided in sufficient detail?
Yes
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Yes
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Yes
References
1. Briceño-León R: [Chagas disease in the Americas: an ecohealth perspective].Cad Saude Publica. 2009; 25 Suppl 1: S71-82 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Sep 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)