Keywords
adverse birth outcomes, pregnancy, preterm birth, small for gestational age, stillbirth, sub-Saharan Africa, Zambia
adverse birth outcomes, pregnancy, preterm birth, small for gestational age, stillbirth, sub-Saharan Africa, Zambia
The often overlapping outcomes of preterm birth (PTB), small for gestational age (SGA), and stillbirth (SB), collectively called ‘adverse birth outcomes’, are responsible for most perinatal morbidity and mortality worldwide.1,2 Low- and middle-income countries bear the overwhelming burden of global PTB, SB, and SGA.3–5 However, reliable classification and estimation of adverse birth outcomes in low-resources settings is challenging because of a number of interrelated factors, including (1) uncertain gestational age dating,6 (2) conflation of fetal growth restriction and PTB into the less useful metric of ‘low birthweight’,3,5 (3) inconsistent thresholds for fetal viability,7 and (4) misclassification of stillbirth and neonatal death.8,9 In many countries, including Zambia, data sources that adequately address these methodological challenges are lacking to the extent that national estimates of adverse birth outcomes must be modeled.3,6,10,11
In sub-Saharan Africa, cohort studies in pregnancy rarely use reliable gestational age dating or clinical phenotyping to classify outcomes. Deliberate clinical phenotyping that characterizes the events that incite parturition (i.e., spontaneous vs. provider-initiated), quantifies maternal and fetal co-morbid conditions, and reliably distinguishes the timing of perinatal death is essential for rigorous classification of adverse birth outcomes.12,13 Accurate estimation of gestational age with fetal ultrasound is also critical. Other dating methods, such as maternal recall of last menstrual period (LMP)14–17, symphisial-fundal height measurement,18 or newborn physical exam19,20 introduce error (and in some cases, bias21).
We established a cohort of 1450 pregnant women and their infants at a tertiary care institution in Lusaka, Zambia, with the goal of better understanding the epidemiological factors and biological mechanisms leading to adverse birth outcomes. This report presents the outcomes of the first phase of this cohort.
The Zambian Preterm Birth Prevention Study (ZAPPS) is an ongoing prospective observational cohort study at the Women and Newborn Hospital of the University Teaching Hospitals (UTH-WNH), the primary referral hospital in Lusaka. Phase 1 of ZAPPS, the subject of this report, recruited and enrolled participants beginning in August 2015 and completed follow-up in June 2018. The sample size for this observational study was initially set at 2000 women, with a target of 250 preterm birth events based on published regional population estimates.4 For budgetary reasons and because the prematurity rate was higher than initially expected, enrollment was stopped in September 2017 after 1450 women had been enrolled. The ZAPPS protocol was developed to align with the Guidelines for Strengthening The Reporting of Observational Studies in Epidemiology (STROBE).22
Pregnant women meeting the following criteria were eligible for enrollment in Phase 1 of the ZAPPS cohort: (1) 18 years of age or older; (2) viable intrauterine singleton or twin gestation; (3) presentation to antenatal care prior to 20 weeks of gestation if HIV-uninfected or 24 weeks if HIV-infected; (4) residing within Lusaka with no plans to relocate during the study follow-up period; (4) willing to provide written, informed consent; (5) willing to allow participation of their infant(s) in the study; (6) willing to be contacted and followed up at home if necessary.
The ZAPPS protocol was approved prior to study initiation and is subjected to annual review by the University of Zambia School of Medicine Biomedical Research Ethics Committee (reference number: 016-04-14) and the University of North Carolina School of Medicine Institutional Review Board (study number: 14-2113). The study also received approval from the Zambian Ministry of Health National Health Research Authority. Each participant provided written informed consent before enrollment.
Full study procedures are described in detail elsewhere.23 Community educators identified potential participants at antenatal care clinics of UTH-WNH and five surrounding clinics in Lusaka, assessing basic eligibility criteria such as age and approximate gestational age. Interested volunteers underwent ultrasound examination per standard of care to determine pregnancy location, fetal viability, number of fetuses, and gestational age by standard biometry (Sonosite M-Turbo, Fuji Sonosite, Bothell, WA). Gestational age was calculated at enrollment by crown-rump length if <14 gestational weeks or by head circumference and femur length if ≥14 weeks. Fetal biometry structures were each measured twice and then averaged to calculate gestational age using INTERGROWTH-21st equations.24,25 Pregnancies below the lower threshold for INTERGROWTH-21st equations were dated by the Hadlock formula.26 Interested women who met preliminary ultrasound eligibility criteria completed an informed consent process in their preferred language of English, Nyanja, or Bemba.
At enrollment, study nurses collected demographic and behavioral information through medical record review and participant interview, and documented a thorough health history including prior pregnancy outcomes. As part of standard antenatal care, participants underwent a physical exam and rapid testing for hemoglobin, urinalysis, syphilis (SD Bioline Syphilis 3.0, Abbott Diagnostics), and HIV (SD Bioline 3.0, Abbott Diagnostics).
After enrollment, participants received routine antenatal care at follow-up visits scheduled at approximately 24 weeks, 32 weeks, and 36 weeks. All participants underwent cervical length measurement in the second trimester (i.e., 14–28 weeks) and fetal growth assessment by biometry in the third trimester.27,28 Cervical length measurements were performed by sonographers with certification in the Cervical Length Education and Review (CLEAR) program. Study nurses staffed the UTH-WNH labor ward full-time and collected detailed information about the clinical course and perinatal outcomes of participants and their infants, including gestational age at birth, neonatal vital status, birthweight, and sex, and assigned a delivery phenotype. For participants who did not deliver at UTH-WNH or were not captured by study staff during their delivery admission, study staff collected perinatal outcomes either in person or by phone. Cohort retention in this analysis was defined as ascertainment of date of delivery.
Primary exposures evaluated included maternal age (years), height (cm), and body mass index (BMI, kg/m2); reported prior preterm birth (nulliparous, parous with no prior PTB, or parous with one or more prior PTB); cervical length (mm) and short cervix (<25mm); gestation (single or twin); hypertension during pregnancy (≥140 systolic or ≥90mmHg diastolic at any antepartum study visit); anemia at enrollment (<10.5g/dL); bacteriuria during pregnancy (1+ leukocyte esterase and/or nitrites at any antepartum study visit); syphilis seropositivity (reactive at enrollment); and HIV seropositivity (reactive at enrollment).
Primary outcomes of this analysis were: PTB, defined as birth between 16 0/7 and 36 6/7 gestational weeks, SGA (newborn weight-for-age <10th percentile by INTERGROWTH-21st norms),29 and SB (delivery of an infant without signs of life ≥16 0/7 weeks). Secondary outcomes included very PTB (birth before 34 0/7 weeks) and very SGA (newborn weight-for-age <3rd percentile). Both PTB and very PTB were further characterized as either spontaneous (spontaneous labor or membrane rupture prior to labor) or provider-initiated (induction of labor or pre-labor cesarean). We differentiated antepartum stillbirth (i.e., fetal heart tones absent on admission or, if not assessed, maceration skin changes present at delivery) from intrapartum stillbirth (i.e., fetal heart tones present on admission or, if not assessed, absence of maceration skin changes at delivery).
We performed descriptive analyses of baseline characteristics and exposures of the cohort, reporting median and interquartile range (IQR) for continuous variables, and frequency and percent for categorical variables. Differences in baseline characteristics between women retained at delivery and those lost to follow-up were evaluated by univariate tests of association.
We summarized parturition phenotype among our retained participants by preterm versus term and spontaneous versus provider-initiated following a standard rubric.12 Among spontaneous PTB, we identified primary maternal, fetal, and/or placental conditions present at the time of delivery. Among provider-initiated PTB, we reported the primary indication for delivery as recorded by the provider. Finally, key individual conditions present and phenotypic clusters were used to classify all PTB, spontaneous PTB, and provider-initiated PTB.
We calculated the incidence of adverse birth outcomes: PTB, spontaneous PTB, very PTB, spontaneous very PTB, SGA, very SGA, and SB among all participants retained at delivery. Twin deliveries in which at least one neonate was SGA or stillborn were classified as having met the respective outcome. Crude associations between key exposures and outcomes were analyzed as risk ratios estimated using Poisson regression analyses with a robust variance.30 Adjusted risk ratios were also estimated using Poisson regression accounting for other key exposures plus maternal age, BMI, estimated gestational age at enrollment, and HIV serostatus at enrollment.
Kaplan-Meier curves were plotted for time-to-delivery with and without a history of prior PTB, short cervix, and twin gestation. We accounted for loss to follow-up by right censoring women at their last study visit (if before delivery) and compared survival between exposure groups by log-rank tests. We also used Cox regression to calculate the hazard of delivery between participants with and without prior PTB, short cervix, and twin gestation, adjusting for maternal age. The proportional-hazards assumption was tested based on Schoenfeld residuals.31 Because of the inherent converging of survival curves in pregnancy at term, we restricted our models to the preterm period by administratively censoring all participants at 37 gestational weeks.32,33
All statistical analyses were performed with Stata version 14 (College Station, TX, USA) and SAS version 9.4 (Cary, NC, USA).
From August 2015 to September 2017, 1784 pregnant women were screened and 1450 (81%) enrolled (Figure 1).23 The median age of enrolled participants was 27 (IQR: 23–32) (Table 1).34 Median estimated gestational age (EGA) at enrollment was 16 weeks; 30% (n=427/1450) were enrolled before 14 completed gestational weeks. Of 1042 (72%) participants who had been pregnant at least once in the past; 19% (n=194/1042) reported a prior miscarriage. Of 992 (68%) with a prior delivery, 41% (n=411) reported a prior PTB. On ultrasound exam, 3% (n=35/1175) had short cervix <25mm, and 3% (n=38/1450) had twin gestation. The prevalence of HIV seropositivity at enrollment was 24% (n=350/1447). Syphilis seropositivity was detected in 5% (70/1342).
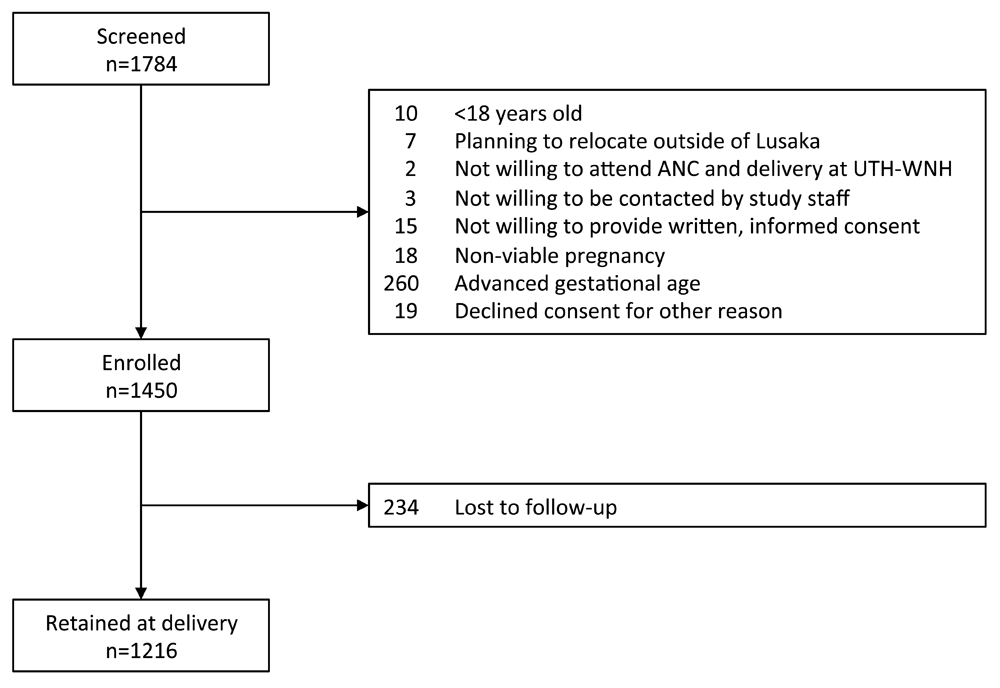
ANC, antenatal care; UTH-WNH, Women and Newborn Hospital of University Teaching Hospital.
| Characteristic | Total enrolled N=1450 | Retained at delivery visit N=1216 (83.9%) | Lost to follow-up N=234 (16.1%) | p | |||
|---|---|---|---|---|---|---|---|
| N | Value % or Median (IQR)* | N | Value % or Median (IQR)* | N | Value % or Median (IQR)* | ||
| Maternal age, years | 1409 | 27 (23–32) | 1192 | 27 (23–32) | 217 | 24 (20–29) | <.001 |
| <20 | 111 | 7.9 | 72 | 6.0 | 39 | 18.0 | |
| 20–34 | 1116 | 79.2 | 956 | 80.2 | 160 | 73.7 | |
| ≥35 | 182 | 12.9 | 164 | 13.8 | 18 | 8.3 | |
| Missing | 41 | 24 | 17 | ||||
| Maternal education, years | 1435 | 12 (9–12) | 1204 | 12 (9–12) | 231 | 9 (7–12) | <.001 |
| None | 26 | 1.8 | 19 | 1.6 | 7 | 3.0 | |
| 0–12 years | 1225 | 85.4 | 1018 | 84.6 | 207 | 89.6 | |
| ≥12 years | 184 | 12.8 | 167 | 13.9 | 17 | 7.4 | |
| Missing | 15 | 12 | 3 | ||||
| Married or cohabiting | 1202 | 83.7 | 1014 | 84.1 | 188 | 81.4 | 0.310 |
| Missing | 13 | 10 | 3 | ||||
| Electricity in home | 1302 | 90.6 | 1105 | 91.6 | 197 | 85.3 | 0.002 |
| Missing | 13 | 10 | 3 | ||||
| Piped drinking water in home | 1340 | 93.3 | 1123 | 93.2 | 217 | 93.9 | 0.678 |
| Missing | 14 | 11 | 3 | ||||
| Toilet facilities in home | <.001 | ||||||
| Flush or Pour | 762 | 53.0 | 667 | 55.3 | 95 | 41.1 | |
| Pit / Latrine / Other | 675 | 47.0 | 539 | 44.7 | 136 | 58.9 | |
| Missing | 13 | 10 | 3 | ||||
| Floor material in home | 0.438 | ||||||
| Natural / rudimentary | 138 | 9.6 | 119 | 9.9 | 19 | 8.2 | |
| Finished | 1299 | 90.4 | 1087 | 90.1 | 212 | 91.8 | |
| Missing | 13 | 10 | 3 | ||||
| Domestic violence in past year | 71 | 5.0 | 58 | 4.9 | 13 | 5.6 | 0.641 |
| Missing | 28 | 26 | 2 | ||||
| Smoking in pregnancy | 8 | 0.6 | 7 | 0.6 | 1 | 0.4 | 0.768 |
| Missing | 24 | 23 | 1 | ||||
| Alcohol use in pregnancy | 124 | 8.7 | 106 | 8.9 | 18 | 7.7 | 0.563 |
| Missing | 25 | 24 | 1 | ||||
| Maternal height at enrollment, cm | 1368 | 156 (160–164) | 1151 | 156 (160–165) | 217 | 156 (160–164) | 0.263 |
| BMI at enrollment, kg/m2 | 1366 | 23.6 (21.2–27.2) | 1149 | 23.9 (21.4–27.6) | 217 | 22.7 (20.7–25.5) | <.001 |
| <18.5 | 71 | 5.2 | 56 | 4.9 | 15 | 6.9 | |
| 18.5–30.0 | 1103 | 80.8 | 919 | 80.0 | 184 | 84.8 | |
| >30.0 | 192 | 14.1 | 174 | 15.1 | 18 | 8.3 | |
| Missing | 84 | 67 | 17 | ||||
| Gravidity | 1450 | 2 (1–4) | 1042 | 2 (1–4) | 408 | 2 (1–3) | 0.003 |
| Primigravida | 408 | 28.1 | 321 | 26.4 | 87 | 37.2 | 0.001 |
| Multigravida | 1042 | 71.9 | 895 | 73.6 | 147 | 62.8 | |
| Prior miscarriage, n=1042 | 0.933 | ||||||
| Multigravida, no prior miscarriage | 848 | 81.4 | 728 | 81.3 | 120 | 81.6 | |
| Multigravida, ≥1 prior miscarriage | 194 | 18.6 | 167 | 18.7 | 27 | 18.4 | |
| Parity | 1450 | 1 (0–2) | 1216 | 1 (0–2) | 234 | 1 (0–2) | 0.004 |
| Nulliparous | 458 | 31.6 | 365 | 30.0 | 93 | 39.7 | 0.003 |
| Parous | 992 | 68.4 | 851 | 70.0 | 141 | 60.3 | |
| Prior PTB, n=992 | 0.224 | ||||||
| Parous, no prior PTB | 581 | 58.6 | 505 | 59.3 | 76 | 53.9 | |
| Parous, ≥1 prior PTB | 411 | 41.4 | 346 | 40.7 | 65 | 46.1 | |
| Prior stillbirth, n=992 | 0.401 | ||||||
| Parous, no prior SB | 780 | 86.1 | 672 | 86.5 | 108 | 83.7 | |
| Parous, ≥1 prior SB | 126 | 13.9 | 105 | 13.5 | 21 | 16.3 | |
| Missing | 86 | 74 | 12 | ||||
| Short cervix < 2.5 cm | 35 | 3.0 | 32 | 3.0 | 3 | 3.2 | 0.899 |
| Missing | 275 | 135 | 140 | ||||
| Twin gestation | 38 | 2.6 | 31 | 2.6 | 7 | 3.0 | 0.698 |
| HIV positive at enrollment | 350 | 24.2 | 304 | 25.0 | 46 | 19.7 | 0.084 |
| Missing | 3 | 2 | 1 | ||||
| Syphilis reactive | 70 | 5.2 | 63 | 5.6 | 7 | 3.1 | 0.142 |
| Missing | 108 | 93 | 15 | ||||
| Hypertensive at enrollment^ | 52 | 3.7 | 46 | 3.9 | 6 | 2.7 | 0.392 |
| Missing | 31 | 21 | 10 | ||||
| Hemoglobin at enrollment, g/dL | 1025 | 12 (11–13) | 854 | 12 (11–13) | 171 | 12 (11–13) | 0.274 |
| <10.5 | 140 | 13.7 | 123 | 14.4 | 17 | 9.9 | |
| Missing | 425 | 362 | 63 | ||||
| Abnormal UA at enrollment† | 69 | 5.0 | 55 | 4.8 | 14 | 6.3 | 0.343 |
| Missing | 79 | 67 | 12 | ||||
| EGA at enrollment, weeks | 1450 | 16.1 (13.3–18.3) | 1216 | 16.0 (13.3–18.3) | 234 | 16.3 (13.3–18.6) | 0.421 |
| <14 | 427 | 29.4 | 362 | 29.8 | 65 | 27.8 | |
Of enrolled participants, 1216 (84%) were retained with delivery date ascertained. Compared to participants lost to follow-up, those retained at delivery were older (median: 27 versus 24 years, p<.001), had more years of education (median: 12 versus 9 years, p<.001), were more likely to have electricity (91% versus 85%, p=.002) and flush or pour toilet facilities at home (55% versus 41%, p<.001), had higher body mass index (23.9 versus 22.7 kg/m2, p<.001), and had higher gravidity (74% versus 63% multigravid, p=.001) and parity (70% versus 60% parous, p=.004).
Frequencies of our outcomes were as follows: 15% PTB (n=181/1216), 8% very PTB (n=92/1216), 18% SGA (n=207/1159), 7% very SGA (n=80/1159), and 4% SB (n=53/1209). Three participants (0.3%) experienced miscarriages before 16 weeks of gestation. Of the pregnancies that ended in SB, 44 (83%) were antepartum and 9 (17%) occurred intrapartum. Among 1159 deliveries within the EGA range for SGA calculation and with birthweight recorded,35 150 (13%) were PTB, 207 (18%) were SGA and 32 (3%) were stillborn (Figure 2).
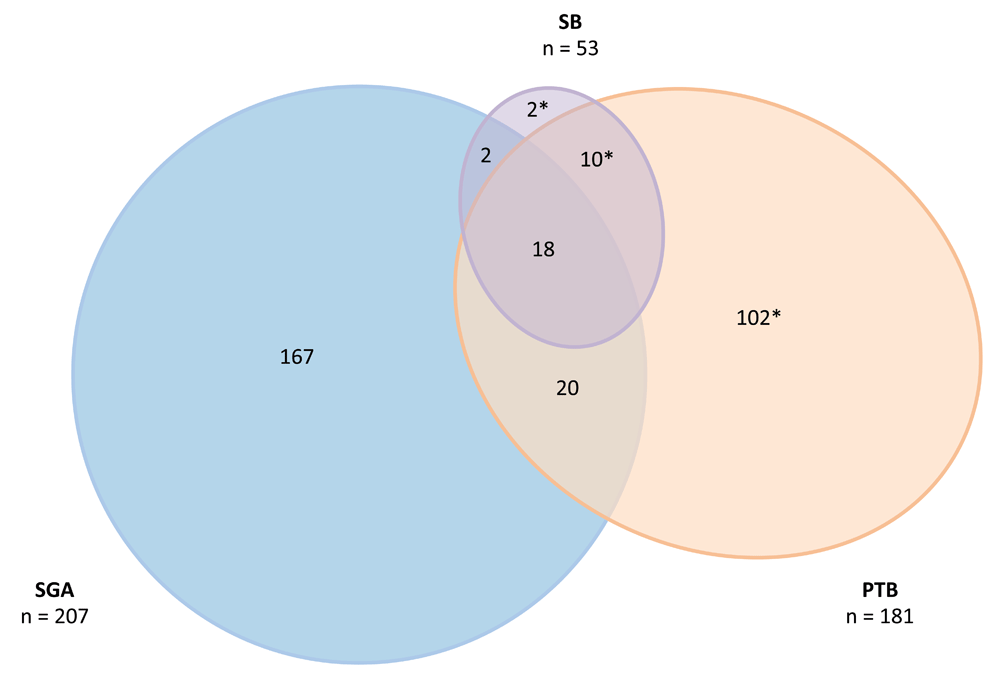
Among ZAPPS cohort participants retained at delivery, 15% (181/1216) were preterm (PTB), 18% (207/1159) were small for gestational age (SGA), and 4% (53/1209) were stillborn (SB). *11 preterm births, one term stillbirth, and 20 preterm stillbirths were either outside the gestational age threshold for INTERGROWTH-21st calculation of SGA,28 or were missing birthweight at delivery. Figure created with: EulerAPE.36
Of 181 total PTB, 120 (66%) occurred spontaneously, 56 (31%) were provider-initiated, and 5 (3%) could not be definitively classified (Figure 3). The most common key conditions present in women with spontaneous PTB (n=120) were HIV infection (n=42, 35%), SB (n=26, 23%), hypertension alone (n=22; 18%), and twin gestation (n=18, 15%); 33 (28%) had no key condition identified. Most provider-initiated preterm deliveries were indicated for SB (n=16, 29%), preeclampsia or eclampsia (n=15, 27%) or hypertension alone (n=4, 7%), or both SB and preeclampsia (n=4, 7%). We identified major phenotypic clusters of PTB, spontaneous PTB, and provider-initiated PTB by presence of maternal, fetal, and/or placental conditions (Table 2).
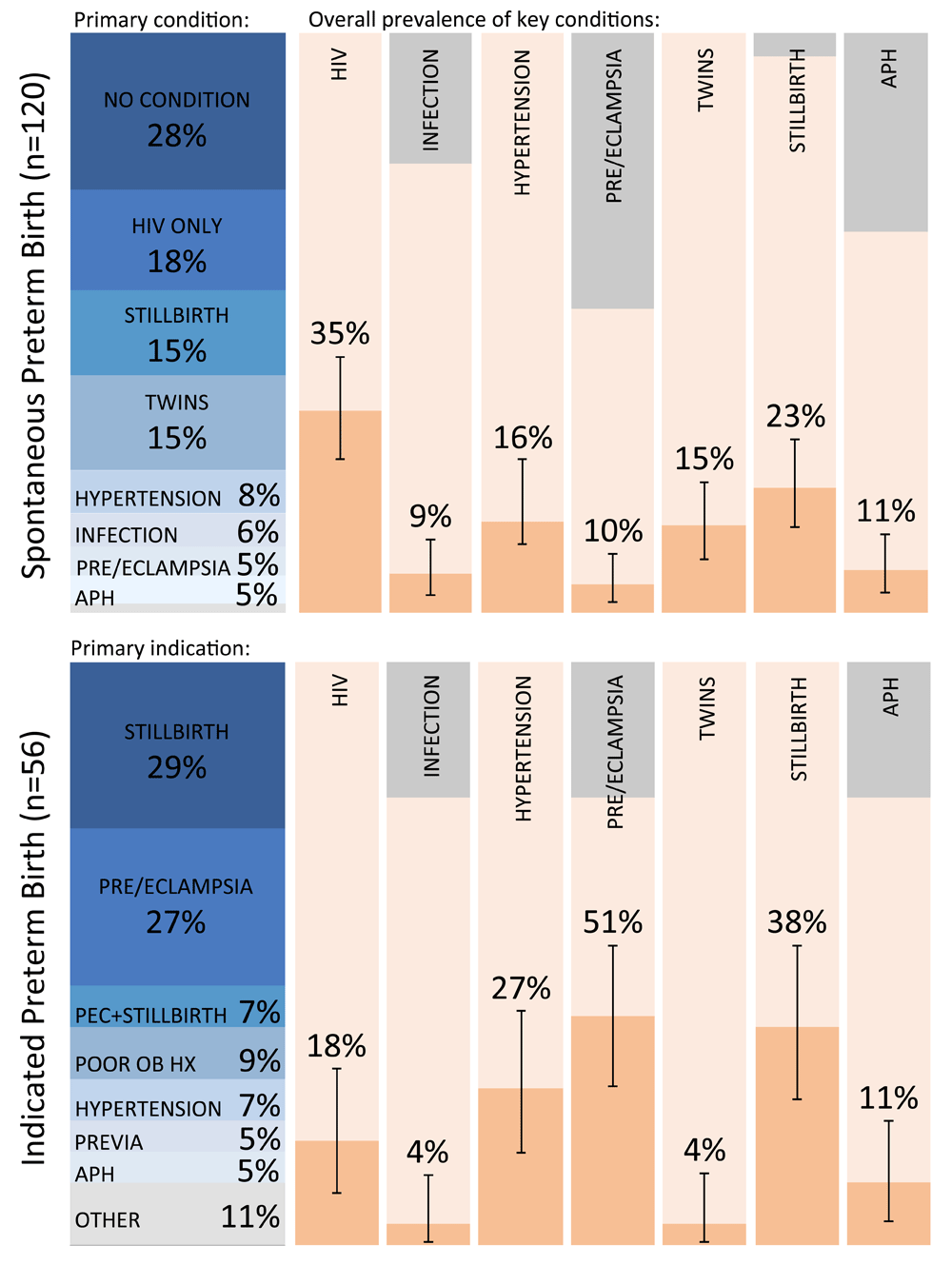
Of participants who underwent preterm delivery (n=181) in the ZAPPS cohort, 120 of them were spontaneous and 56 were indicated. This figure presents the frequencies of primary conditions present in spontaneous preterm deliveries, primary indications for indicated preterm deliveries, and the overall frequency with 95% confidence intervals of key conditions in each group. Gray bars represent missing values. PTB, preterm birth; PEC, preeclampsia; EC; eclampsia; APH, antepartum hemorrhage; OB HX, obstetrical history.
| All preterm birth N (%)* | Spontaneous N (%)† | Provider-initiated N (%)† | ||||
|---|---|---|---|---|---|---|
| All preterm | 181 | 100 | 120 | 68 | 56 | 32 |
| Phenotypic clusters | ||||||
| No significant clinical conditions | 41 | 23 | 33 | 87 | 5 | 13 |
| Maternal condition(s) only | 60 | 33 | 40 | 67 | 20 | 33 |
| Fetal condition(s) only | 27 | 15 | 21 | 81 | 5 | 19 |
| Placental condition(s) only | 4 | 2 | 1 | 25 | 3 | 75 |
| Maternal and fetal conditions | 37 | 20 | 17 | 47 | 19 | 53 |
| Maternal and placental conditions | 6 | 3 | 3 | 50 | 3 | 50 |
| Fetal and placental conditions | 1 | 1 | 1 | 100 | 0 | 0 |
| Maternal, fetal, and placental conditions | 5 | 3 | 4 | 80 | 1 | 20 |
| Significant maternal conditions | 108 | 60 | 64 | 60 | 43 | 40 |
| HIV infection | 53 | 29 | 42 | 81 | 10 | 19 |
| Urinary tract infection, n=41 | 9 | 22 | 7 | 78 | 2 | 22 |
| Clinical chorioamnionitis, n=120 | 1 | 1 | 1 | 100 | 0 | 0 |
| Diabetes (mellitus or gestational), n=179 | 5 | 3 | 1 | 20 | 4 | 80 |
| Hypertension | 34 | 19 | 19 | 56 | 15 | 44 |
| Preeclampsia, n=107 | 23 | 21 | 6 | 26 | 17 | 74 |
| Eclampsia, n=125 | 5 | 4 | 0 | 0 | 5 | 100 |
| Significant fetal conditions | 70 | 39 | 43 | 63 | 25 | 37 |
| Twin gestation | 21 | 12 | 18 | 90 | 2 | 10 |
| Stillbirth, n=176 | 48 | 27 | 26 | 55 | 21 | 45 |
| Fetal growth restriction | 2 | 1 | 0 | 0 | 2 | 100 |
| Fetal distress | 1 | 1 | 0 | 0 | 1 | 100 |
| Polyhydramnios | 1 | 1 | 0 | 0 | 1 | 100 |
| Oligohydramnios | 1 | 1 | 0 | 0 | 1 | 100 |
| Significant placental conditions | 16 | 9 | 9 | 56 | 7 | 44 |
| Placental abruption | 15 | 8 | 9 | 60 | 6 | 40 |
| Placenta previa | 4 | 2 | 0 | 0 | 4 | 100 |
Maternal age ≥35, prior PTB, short cervix, twin gestation, antenatal hypertension, and EGA at enrollment <14 weeks were associated with PTB (Table 3). Overall, these associations were stable or strengthened when restricting the outcome to spontaneous PTB and to very PTB (Table 4). Although associated with PTB, antenatal hypertension did not significantly predict spontaneous phenotypes of PTB. In multivariable regression models adjusting for maternal age, BMI, EGA at enrollment, and HIV status at enrollment, participants with prior PTB (aRR 1.88; 95% CI 1.32–2.68), short cervix (aRR 2.62; 95% CI 1.68–4.09), twin gestation (aRR 5.22; 95% CI 3.67–7.43), and antenatal hypertension (aRR 2.04; 95% CI 1.43–2.91) had increased risk of PTB (Table 2). The associations between the exposures of prior PTB, short cervix, and twin gestation with PTB were stable or strengthened when restricting the outcome to spontaneous phenotypes and very PTB (Table 4). The risk of PTB decreased with increasing cervical length (RR 0.58 per cm; 95% CI 0.46–0.73) (Figure 4).
| Exposure | Preterm birth 16 to <37 weekss | Spontaneous preterm birth 16 to <37 weeks | Small for gestational age | Stillbirth | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | events | % | RR | 95% CI | aRR* | 95% CI | n | events | % | RR | 95% CI | aRR* | 95% CI | n | events | % | RR | 95% CI | aRR‡ | 95% CI | n | events | % | RR | 95% CI | aRR‡ | 95% CI | |
| Age at enrollment, years | ||||||||||||||||||||||||||||
| <20 | 72 | 5 | 6.9 | 1.00 | 70 | 3 | 4.3 | 1.00 | 70 | 13 | 18.6 | 1.00 | 72 | 0 | 0.0 | - | ||||||||||||
| 20–34 | 953 | 144 | 15.1 | 2.18 | 0.92– 5.14 | 950 | 103 | 10.8 | 2.53 | 0.82– 7.77 | 911 | 157 | 17.2 | 0.93 | 0.56– 1.55 | 954 | 39 | 4.1 | 1.00 | |||||||||
| ≥35 | 164 | 31 | 18.9 | 2.72 | 1.10– 6.72 | 164 | 13 | 7.9 | 1.85 | 0.54– 6.29 | 155 | 33 | 21.3 | 1.15 | 0.64– 2.04 | 159 | 14 | 8.8 | 2.15 | 1.20– 3.88 | ||||||||
| BMI at enrollment, kg/m2 | ||||||||||||||||||||||||||||
| <18.5 | 56 | 12 | 21.4 | 1.00 | 56 | 10 | 17.9 | 1.00 | 53 | 10 | 18.9 | 1.00 | 55 | 1 | 1.8 | 1.00 | ||||||||||||
| 18.5–30.0 | 916 | 136 | 14.9 | 0.69 | 0.41– 1.17 | 912 | 90 | 9.9 | 0.55 | 0.30– 1.00 | 876 | 172 | 19.6 | 1.04 | 0.59– 1.85 | 913 | 41 | 4.5 | 2.47 | 0.35– 17.6 | ||||||||
| >30.0 | 174 | 20 | 11.5 | 0.54 | 0.28– 1.03 | 173 | 10 | 5.8 | 0.32 | 0.14– 0.74 | 168 | 18 | 10.7 | 0.57 | 0.28– 1.15 | 173 | 7 | 4.1 | 2.23 | 0.28– 17.7 | ||||||||
| Prior PTB | ||||||||||||||||||||||||||||
| Nulliparous | 364 | 40 | 11.0 | 1.13 | 0.76– 1.68 | 0.93 | 0.60– 1.43 | 363 | 25 | 6.9 | 1.23 | 0.73– 2.08 | 0.86 | 0.47– 1.55 | 345 | 73 | 21.2 | 1.42 | 1.06– 1.91 | 1.36 | 0.98– 1.87 | 363 | 8 | 2.2 | 0.62 | 0.27– 1.40 | 0.95 | 0.36– 2.50 |
| Parous, no prior PTB | 504 | 49 | 9.7 | 1.00 | 1.00 | 502 | 28 | 5.6 | 1.00 | 1.00 | 491 | 73 | 14.9 | 1.00 | 1.00 | 503 | 18 | 3.6 | 1.00 | 1.00 | ||||||||
| Parous, ≥1 prior PTB | 345 | 92 | 26.7 | 2.74 | 1.99– 3.77 | 1.88 | 1.32– 2.68 | 343 | 67 | 19.5 | 3.5 | 2.30– 5.33 | 2.62 | 1.69– 4.06 | 323 | 61 | 18.9 | 1.27 | 0.93– 1.73 | 1.12 | 0.82– 1.54 | 343 | 27 | 7.9 | 2.20 | 1.23– 3.93 | 1.63 | 0.78– 3.40 |
| Cervical length | ||||||||||||||||||||||||||||
| ≥2.5cm | 1049 | 137 | 13.1 | 1.00 | 1.00 | 1045 | 91 | 8.7 | 1.00 | 1.00 | 1021 | 181 | 17.7 | 1.00 | 1046 | 35 | 3.4 | 1.00 | 1.00 | |||||||||
| <2.5cm | 32 | 16 | 50.0 | 3.83 | 2.62– 5.60 | 2.62 | 1.68– 4.09 | 32 | 9 | 28.1 | 3.23 | 1.79– 5.81 | 1.95 | 1.01– 3.77 | 31 | 9 | 29.0 | 1.64 | 0.93– 2.89 | 32 | 6 | 18.8 | 5.60 | 2.54– 12.4 | 6.42 | 2.56– 16.1 | ||
| Gestation | ||||||||||||||||||||||||||||
| Single | 1182 | 160 | 13.5 | 1.00 | 1.00 | 1178 | 102 | 8.7 | 1.00 | 1.00 | 1128 | 191 | 16.9 | 1.00 | 1.00 | 1177 | 51 | 4.3 | 1.00 | |||||||||
| Twin | 31 | 21 | 67.7 | 5.00 | 3.77– 6.64 | 5.22 | 3.67– 7.43 | 30 | 18 | 60.0 | 6.93 | 4.90– 9.80 | 7.86 | 5.37– 11.5 | 31 | 16 | 51.6 | 3.05 | 2.12– 4.39 | 2.75 | 1.81– 4.18 | 31 | 2 | 6.5 | 1.49 | 0.38– 5.85 | ||
| HIV serostatus at enrollment | ||||||||||||||||||||||||||||
| Negative | 908 | 128 | 14.1 | 1.00 | 1.00 | 904 | 78 | 8.6 | 1.00 | 1.00 | 871 | 152 | 17.5 | 1.00 | 1.00 | 906 | 37 | 4.1 | 1.00 | 1.00 | ||||||||
| Positive | 303 | 53 | 17.5 | 1.24 | 0.93– 1.66 | 1.17 | 0.85– 1.62 | 302 | 42 | 13.9 | 1.61 | 1.13– 2.29 | 1.36 | 0.91– 2.03 | 286 | 55 | 19.2 | 1.10 | 0.83– 1.46 | 1.09 | 0.80– 1.47 | 301 | 16 | 5.3 | 1.30 | 0.73– 2.31 | 1.29 | 0.65– 2.56 |
| Syphilis | ||||||||||||||||||||||||||||
| Non-reactive | 1057 | 159 | 15.0 | 1.00 | 1053 | 109 | 10.4 | 1.00 | 1006 | 184 | 18.3 | 1.00 | 1053 | 41 | 3.9 | 1.00 | 1.00 | |||||||||||
| Reactive | 63 | 9 | 14.3 | 0.95 | 0.51– 1.77 | 63 | 3 | 4.8 | 0.46 | 0.15– 1.41 | 63 | 13 | 20.6 | 1.13 | 0.68– 1.86 | 63 | 6 | 9.5 | 2.45 | 1.08– 5.55 | 2.34 | 0.91– 6.04 | ||||||
| Blood pressure during pregnancy | ||||||||||||||||||||||||||||
| Normotensive | 1072 | 145 | 13.5 | 1.00 | 1.00 | 1067 | 106 | 9.9 | 1.00 | 1025 | 173 | 16.9 | 1.00 | 1.00 | 1068 | 42 | 3.9 | 1.00 | 1.00 | |||||||||
| Hypertensive‡s | 141 | 36 | 25.5 | 1.89 | 1.37– 2.60 | 2.04 | 1.43– 2.91 | 141 | 14 | 9.9 | 1.00 | 0.59– 1.70 | 134 | 34 | 25.4 | 1.50 | 1.09– 2.07 | 1.62 | 1.16– 2.26 | 141 | 11 | 7.8 | 1.98 | 1.05– 3.76 | 1.83 | 0.84– 3.96 | ||
| Hemoglobin at enrollment | ||||||||||||||||||||||||||||
| ≥10.5 g/dL | 730 | 111 | 15.2 | 1.00 | 727 | 70 | 9.6 | 1.00 | 700 | 125 | 17.9 | 1.00 | 726 | 30 | 4.1 | 1.19 | 0.51– 2.80 | |||||||||||
| <10.5 g/dL | 121 | 21 | 17.4 | 1.14 | 0.75– 1.75 | 121 | 15 | 12.4 | 1.29 | 0.76– 2.17 | 113 | 20 | 17.7 | 0.99 | 0.65– 1.52 | 122 | 6 | 4.9 | 1.00 | |||||||||
| UA during pregnancy | ||||||||||||||||||||||||||||
| Normal | 907 | 125 | 13.8 | 1.00 | 903 | 82 | 9.1 | 1.00 | 880 | 168 | 19.1 | 1.00 | 903 | 32 | 3.5 | 1.00 | ||||||||||||
| Abnormal^ | 189 | 31 | 16.4 | 1.19 | 0.83– 1.71 | 189 | 20 | 10.6 | 1.17 | 0.73– 1.85 | 182 | 26 | 14.3 | 0.75 | 0.51– 1.10 | 191 | 6 | 3.1 | 0.89 | 0.38– 2.09 | ||||||||
| EGA at enrollment, weeks | ||||||||||||||||||||||||||||
| <14 | 360 | 72 | 20.0 | 1.00 | 358 | 45 | 12.6 | 1.00 | 333 | 62 | 18.6 | 1.00 | 357 | 20 | 5.6 | 1.00 | ||||||||||||
| ≥14 | 853 | 109 | 12.8 | 0.64 | 0.49– 0.84 | 850 | 75 | 8.8 | 0.70 | 0.50– 0.99 | 826 | 145 | 17.6 | 0.94 | 0.72– 1.23 | 851 | 33 | 3.9 | 0.69 | 0.40– 1.19 | ||||||||
* Risk ratios calculated via Poisson regression with robust error variance. Multivariable model estimates of adjusted risk ratios include other exposure variables listed and all models adjusted for: maternal age, maternal BMI, and EGA at enrollment as continuous variables.
| Exposure | Very preterm birth 16 to <34 weeks | Spontaneous very preterm birth 16 to <34 weeks | Very small for gestational age | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | events | % | RR | 95% CI | aRR* | 95% CI | n | events | % | RR | 95% CI | aRR* | 95% CI | n | events | % | RR | 95% CI | aRR* | 95% CI | |
| Age at enrollment, years | |||||||||||||||||||||
| <20 | 72 | 3 | 4.2 | 1.00 | 71 | 2 | 2.8 | 1.00 | 70 | 7 | 10.0 | 1.00 | |||||||||
| 20–34 | 953 | 75 | 7.9 | 1.89 | 0.61–5.84 | 950 | 50 | 5.3 | 1.87 | 0.46–7.53 | 913 | 55 | 6.0 | 1.66 | 0.79–3.51 | ||||||
| ≥35 | 164 | 13 | 7.9 | 1.90 | 0.56–6.48 | 164 | 6 | 3.7 | 1.30 | 0.27–6.28 | 155 | 17 | 11.0 | 1.82 | 1.09–3.05 | ||||||
| BMI at enrollment, kg/m2 | |||||||||||||||||||||
| <18.5 | 56 | 4 | 7.1 | 1.00 | 56 | 3 | 5.4 | 1.00 | 53 | 8 | 15.1 | 1.00 | |||||||||
| 18.5–30.0 | 916 | 72 | 7.9 | 1.10 | 0.42–2.90 | 913 | 46 | 5.0 | 0.94 | 0.30–2.93 | 879 | 60 | 6.8 | 0.45 | 0.23–0.90 | ||||||
| >30.0 | 174 | 9 | 5.2 | 0.72 | 0.23–2.27 | 173 | 5 | 2.9 | 0.54 | 0.13–2.19 | 167 | 10 | 6.0 | 0.40 | 0.17–0.95 | ||||||
| Prior PTB | |||||||||||||||||||||
| Nulliparous | 364 | 22 | 6.0 | 1.45 | 0.81–2.60 | 1.10 | 0.55–2.21 | 364 | 14 | 3.9 | 1.61 | 0.75–3.44 | 0.88 | 0.34–2.31 | 345 | 32 | 9.3 | 1.98 | 1.18–3.32 | 1.92 | 1.12–3.32 |
| Parous, no prior PTB | 504 | 21 | 4.2 | 1.00 | 1.00 | 502 | 12 | 2.4 | 1.00 | 1.00 | 491 | 23 | 4.7 | 1.00 | 1.00 | ||||||
| Parous, ≥1 prior PTB | 345 | 49 | 14.2 | 3.41 | 2.08–5.58 | 2.27 | 1.28–4.04 | 343 | 33 | 9.6 | 4.02 | 2.11–7.68 | 2.89 | 1.39–5.98 | 323 | 25 | 7.7 | 1.65 | 0.95–2.86 | 1.39 | 0.76–2.53 |
| Cervical length | |||||||||||||||||||||
| ≥2.5cm | 1049 | 63 | 6.0 | 1.00 | 1.00 | 1046 | 39 | 3.7 | 1.00 | 1.00 | 1022 | 68 | 6.7 | 1.00 | 1.00 | ||||||
| <2.5cm | 32 | 12 | 37.5 | 6.24 | 3.76–10.4 | 3.97 | 2.15–7.33 | 32 | 7 | 21.9 | 5.87 | 2.84–12.1 | 3.19 | 1.35–7.55 | 31 | 5 | 16.1 | 2.42 | 1.05–5.59 | 2.06 | 0.88–4.82 |
| Gestation | |||||||||||||||||||||
| Single | 1182 | 81 | 6.9 | 1.00 | 1.00 | 1179 | 51 | 4.3 | 1.00 | 1.00 | 1130 | 74 | 6.6 | 1.00 | 1.00 | ||||||
| Twin | 31 | 11 | 35.5 | 5.18 | 3.08–8.70 | 5.18 | 2.75–9.77 | 30 | 8 | 26.7 | 6.16 | 3.21–11.8 | 7.53 | 3.58–15.9 | 31 | 6 | 19.4 | 2.96 | 1.39–6.27 | 2.71 | 1.12–6.57 |
| HIV serostatus at enrollment | |||||||||||||||||||||
| Negative | 908 | 67 | 7.4 | 1.00 | 1.00 | 905 | 41 | 4.5 | 1.00 | 1.00 | 871 | 61 | 7.0 | 1.00 | 1.00 | ||||||
| Positive | 303 | 25 | 8.3 | 1.12 | 0.72–1.74 | 1.20 | 0.71–2.01 | 302 | 18 | 6.0 | 1.32 | 0.77–2.26 | 1.35 | 0.68–2.66 | 286 | 19 | 6.6 | 0.95 | 0.58–1.56 | 0.86 | 0.48–1.53 |
| Syphilis | |||||||||||||||||||||
| Non-reactive | 1057 | 81 | 7.7 | 1.00 | 1054 | 53 | 5.0 | 1.00 | 1009 | 71 | 7.0 | 1.00 | |||||||||
| Reactive | 63 | 5 | 7.9 | 1.04 | 0.44–2.46 | 63 | 2 | 3.2 | 0.63 | 0.16–2.53 | 63 | 6 | 9.5 | 1.35 | 0.61–2.99 | ||||||
| Blood pressure during pregnancy | |||||||||||||||||||||
| Normotensive | 1072 | 78 | 7.3 | 1.00 | 1068 | 55 | 5.2 | 1.00 | 1025 | 65 | 6.3 | 1.00 | 1.00 | ||||||||
| Hypertensive‡ | 141 | 14 | 9.9 | 1.36 | 0.79–2.34 | 141 | 4 | 2.8 | 0.55 | 0.20–1.50 | 134 | 15 | 11.2 | 1.77 | 1.04–3.00 | 1.68 | 0.92–3.06 | ||||
| Hemoglobin at enrollment | |||||||||||||||||||||
| ≥10.5 g/dL | 730 | 60 | 8.2 | 1.00 | 727 | 35 | 4.8 | 1.00 | 703 | 46 | 6.5 | 1.00 | |||||||||
| <10.5 g/dL | 121 | 7 | 5.8 | 0.70 | 0.33–1.50 | 121 | 5 | 4.1 | 0.86 | 0.34–2.15 | 113 | 5 | 4.4 | 0.68 | 0.27–1.67 | ||||||
| UA during pregnancy | |||||||||||||||||||||
| Normal | 907 | 59 | 6.5 | 1.00 | 904 | 37 | 4.1 | 1.00 | 881 | 68 | 7.7 | 1.00 | |||||||||
| Abnormal^ | 189 | 14 | 7.4 | 1.14 | 0.65–2.00 | 189 | 7 | 3.7 | 0.81 | 0.41–2.00 | 183 | 10 | 5.5 | 0.71 | 0.37–1.35 | ||||||
| EGA at enrollment, weeks | |||||||||||||||||||||
| <14 | 360 | 39 | 10.8 | 1.00 | 358 | 26 | 7.3 | 1.00 | 333 | 25 | 7.5 | 1.00 | |||||||||
| ≥14 | 853 | 53 | 6.2 | 0.57 | 0.39–0.85 | 851 | 33 | 3.9 | 0.92 | 0.86–0.99 | 828 | 55 | 6.6 | 0.88 | 0.56–1.40 | ||||||
* Risk ratios calculated via Poisson regression with robust error variance. Multivariable models include other exposure variables listed and adjusted for: maternal age, maternal BMI, and EGA at enrollment.
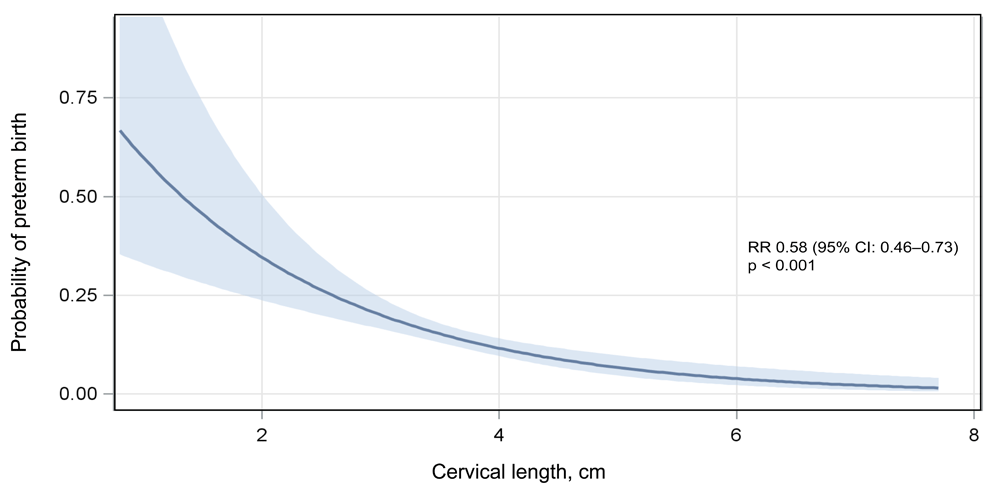
Among ZAPPS cohort participants with a cervical length measured by ultrasound in the second trimester (n=1081), the probability of preterm birth <37 weeks decreased with increasing cervical length. PTB, preterm birth; RR, relative risk; CI, confidence interval.
Nulliparity, twin gestation, and antenatal hypertension were each associated with SGA in univariate analysis, and older age, low BMI, nulliparity, short cervix, twin gestation, and antenatal hypertension were associated with very SGA. In multivariable analysis, twin gestation (aRR 2.75; 95% CI 1.81–4.18) and antenatal hypertension (aRR 1.62; 95% CI 1.16–2.26) were associated with an increased risk of SGA; nulliparity was marginally associated with SGA (aRR 1.36; 95% CI 0.98–1.87). Nulliparity (aRR 1.92; 95% CI 1.12–3.32) and twin gestation (aRR 2.71, 95% CI 1.12–6.57) were associated with very SGA. Maternal height was not associated with SGA or very SGA in univariate analyses.
Finally, older maternal age, prior PTB, short cervix, syphilis seropositivity, and antenatal hypertension were individually associated with an elevated risk of SB (Table 3). In multivariable analysis, short cervix predicted SB (aRR 6.42; 95% CI 2.56–16.1), while syphilis was only marginally associated (aRR 2.34; 95% CI 0.91–6.04).
Elevated risks of PTB among women with prior PTB, short cervix, and twin gestation were supported by survival analyses, with log-rank tests of association demonstrating significant differences between groups of each variable (Figure 5; Table 5). In proportional hazards models adjusted for maternal age at enrollment, participants with prior PTB, short cervix, and twin gestation had significantly higher hazards of delivering before 37 gestational weeks compared to parous women with no prior PTB, women with cervical lengths ≥25mm, or with single gestations. Participants with increasing numbers of prior preterm births demonstrated increasing hazard ratios of delivering preterm.
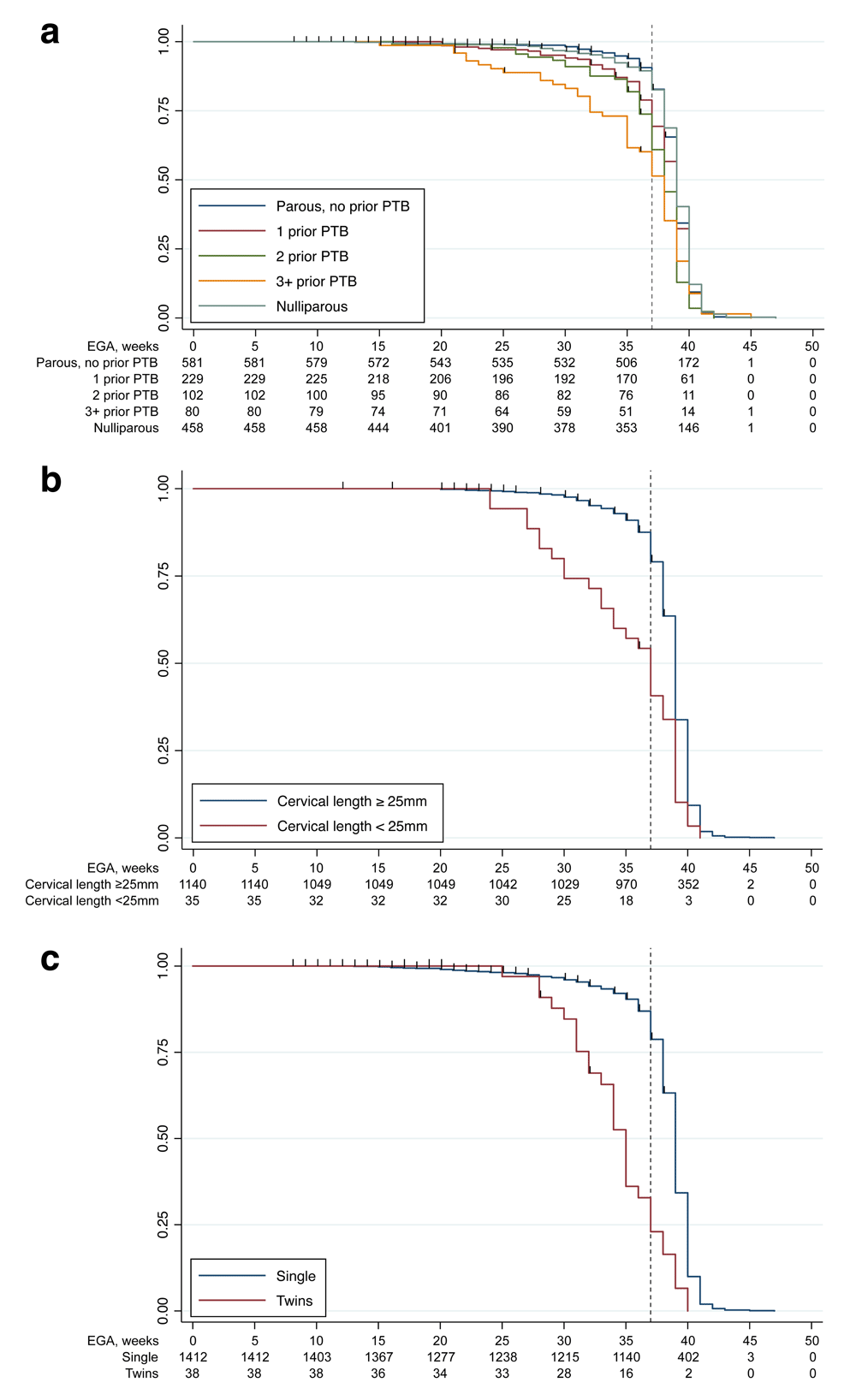
Kaplan-Meier survival curves by (a) prior preterm birth, (b) short cervix (<25mm), and (c) twin gestation. Survival curves are presented for participants with increasing numbers of prior preterm birth, those with cervical length <25mm compared to ≥25 mm, and those with twin compared to singleton gestation. The dashed vertical line represents a gestational age of 37 weeks, the threshold for preterm versus term delivery. EGA, estimated gestational age; PTB, preterm birth.
| Log-rank | Cox proportional hazards | Schoenfeld residual test | |||||
|---|---|---|---|---|---|---|---|
| p | HR* | 95% CI | p | rho | Χ2 | p | |
| Prior preterm birth | global | 3.23 | 0.66 | ||||
| Parous, no prior | <.001^ | ref | ref | ||||
| Parous, 1 prior | 2.21 | 1.47– 3.33 | <.001 | -0.01 | 0.01 | 0.92 | |
| Parous, 2 prior | 2.79 | 1.70– 4.58 | <.001 | 0.00 | 0.00 | 0.95 | |
| Parous, 3+ prior | 4.70 | 2.94– 7.53 | <.001 | -0.11 | 2.33 | 0.13 | |
| Nulliparous | - | 1.16 | 0.75– 1.78 | 0.51 | -0.06 | 0.80 | 0.37 |
| Cervical length | global | 4.42 | 0.11 | ||||
| ≥ 25mm | <.001 | ref | ref | ||||
| <25mm | 5.19 | 3.09– 8.74 | <.001 | -0.15 | 3.26 | 0.07 | |
| Gestation | global | 3.19 | 0.20 | ||||
| Singleton | <.001 | ref | ref | ||||
| Twin | 6.70 | 4.25– 10.60 | <.001 | 0.13 | 3.19 | 0.07 | |
We present the primary results of the ZAPPS pregnancy cohort, established to evaluate the risk factors associated with adverse birth outcomes in Lusaka, Zambia. This study was notable for enrollment of pregnant women at early presentation to antenatal care, gestational age determination by early ultrasound, universal cervical length screening, comprehensive and uniform antenatal and postpartum care, and clinical phenotyping of birth outcomes. Our analyses revealed strong risks of prior preterm birth, short mid-trimester cervical length, and twin gestation on incident preterm birth, and these risks were supported by analyses of pregnancy ‘survival’ to term. We also report increased risks of small-for-gestational-age infants among nulliparous women and women with twin gestation, and of stillbirth among those with short cervix.
The proportion of gravidas who deliver before term varies significantly across individual studies and national estimates in sub-Saharan Africa. The most recent global report estimated a PTB rate of 12% in Zambia based on modeled regional estimates.6 In contrast, a census accounting of 237,219 public sector births over 6 years in Lusaka - where the vast majority of pregnancies are dated by last menstrual period - classified 46% of singleton deliveries as preterm.37,38 In Zambia, obstetrical ultrasound is rare and the reliance on maternal recall of LMP alone substantially over-estimates preterm birth rates,17,39 an inaccuracy that worsens with later presentation to care.21 We report PTB based on ultrasound gestational age dating and prospectively ascertained delivery outcomes such that our data are likely more accurate than reports that rely on LMP recall or regional models.
The distinction of preterm parturition as spontaneously occurring versus provider-initiated is important but rarely reported from national surveillance or clinical research data in low-resource settings. Deliveries that are preceded by spontaneous labor or membrane rupture are phenotypically distinct from those that are induced medically or surgically for complications such as preeclampsia, fetal demise, or other maternal or fetal conditions.12,40–43 Further classification based on primary conditions present in spontaneous PTB and the primary indications for provider-initiated PTB is based on a standardized rubric proposed to elucidate phenotypic clusters of PTB.12 An understanding of prevailing phenotypes can direct research, policy, and preventive interventions towards regional and population-specific needs.13,44,45 While our cohort is limited by a small number of PTB events (n=181), we were able to classify nearly all (i.e., 97%) as either spontaneous or provider-initiated, and to identify the primary complications and phenotypic characteristics of each. Further granularity and generalizability requires a larger sample size, signaling a need for future high-quality obstetrical research on a greater scale.
As with PTB classification, identifying infants born SGA requires accurate gestational age estimation, which can be at best imprecise, and at worst biased, when based solely on LMP.21 The incidence of SGA in our cohort (18%) was modestly higher than a recent estimate of SGA in Zambia of 13%, again modeled from published rates in other neighboring countries because of scarcity of data from Zambia itself.5 In comparison to the ZAPPS cohort, in which older maternal age, low BMI, nulliparity, twin gestation, and antenatal hypertension predicted either SGA or very SGA, a study among over 19,000 singletons in Tanzania identified younger maternal age, height, and nulliparity as strong risk factors for SGA.46 The WHO Multi-country Survey on Maternal and Newborn Health found nulliparity and hypertensive disorders to indicate higher risk of preterm SGA and hypertensive disorders, sociodemographic factors, and anemia to predict term SGA.47 With a much smaller sample size and fewer outcomes compared to these two studies, we were not able to differentiate our outcome by preterm vs. term SGA due to low statistical precision for stratified associations with key risk factors. Due to this low precision, we are not able to discern whether or not SGA outcomes were modified by gestational age at delivery. However, both of these studies relied on reported LMP to estimate gestational age at delivery, which itself may have introduced error. Whether growth restriction is a distinct pathological process before 37 weeks compared to after 37 weeks is unclear. Finally, while the INTERGROWTH-21st Project48 intended to define universal fetal growth and newborn weight standards derived from an extensive multi-ethnic sample of women with adequate antenatal care and nutrition, its widespread use over ethnicity-specific or customized standards has been disputed.42,49–55 Despite this, we chose to define SGA in our cohort based on INTERGROWTH-21st standards since local standards that include all pregnancies affected by undernutrition and/or pregnancy comorbidities tend to identify only the severest 10% of cases by definition.
Stillbirth, a composite outcome comprising antepartum and intrapartum fetal death, is particularly understudied in low-resource settings. The true global burden of stillbirth and its underlying causes are poorly classified due to inconsistent fetal viability limits and imperfect classification of neonatal death versus stillbirth, limited resources for case investigations, under-reporting of home births that result in perinatal death, and inadequate national and regional reporting of identified cases.3 Indeed, recent global and regional estimates of stillbirth included just 17% of its datapoints from sub-Saharan Africa and south Asia, regions that bear 77% of the global burden.3 Data from the recent Zambia Demographic and Health Survey reported a rate of stillbirth, defined as fetal death over 7 months’ gestation, as 1.3% among 13,563 births reported, with equal rates outside Lusaka province as within.56 This is similar to estimates from a Global Network study in Zambia, in which 2% of women enrolled delivered stillbirths.9 The slightly higher proportion of deliveries that resulted in stillbirth in the ZAPPS cohort, at least partly attributable to a broader gestational age range, was reflected in the ZEPRS database, in which 6% of 66,395 deliveries at UTH resulted in stillbirth.38 However, over half of stillbirths in ZEPRS and 67% in a Global Network study in Zambia were classified as intrapartum, compared to less than 20% in the ZAPPS cohort. These disparities may result from differential classification; it is standard practice outside of our study to classify stillbirths solely by neonatal skin maceration at delivery, particularly in the absence of but even despite the presence of documented fetal heart activity during labor.9 Indeed, previous studies have demonstrated that reliance on observed skin maceration alone can over-estimate stillbirth proportions attributable to the intrapartum period.57
This study has several limitations, many of which have been noted previously.23 First, 16% of participants were lost to follow-up. While this is commensurate with other longitudinal pregnancy cohort studies in the region,58,59 error may be introduced if outcomes are not missing at random.60 Women lost to follow-up were younger, more likely to be primigravida and nulliparous, had lower BMIs, and had multiple lower measures of socioeconomic status; many of these characteristics were risk factors for at least one adverse outcome. Further, 250 (21%) of the retained participants either did not deliver at the study hospital or delivered at a time when ZAPPS staff were not present, requiring delivery outcomes to be ascertained by record review and/or participant report (it is worth noting that we found no difference in frequencies of outcomes between deliveries attended by ZAPPS staff versus those that were not; see Underlying data). Second, our data have noted missingness of key antenatal test results at baseline (i.e., hemoglobin, syphilis, and urinalysis) because tests were not routinely repeated nor results recorded in our database if performed at the recruitment clinic before enrollment. Of these test results, only syphilis was associated with an outcome (stillbirth), but we cannot determine with certainty whether missingness introduced bias or simply reduced statistical power. Third, while the ZAPPS study recruits from several surrounding primary clinics, it is based at a tertiary referral hospital and many of our participants were drawn from this higher-risk pool. We note high prevalence of prior PTB, miscarriage, and stillbirth, and high HIV and syphilis seropositivity, which may have resulted from self-selection of high-risk women into a cohort study investigating adverse birth outcomes. It is likely that this resulted in an over-representation of outcomes, but less likely to have also introduced a biased association with identified risk factors.
In summary, the ZAPPS cohort study demonstrates high prevalence of antenatal comorbidities and identifies a number of factors associated with increased risks of preterm birth, small-for-gestational-age infants, and stillbirth. This is the first study of its kind to be conducted in Zambia, and one of the largest on the African continent. An understanding of the true global scope of adverse birth outcomes will require consistent definitions, meticulous ascertainment, and systematic reporting that has eluded those settings where the burden of these outcomes is highest. In the absence of sophisticated registry infrastructure, large pregnancy cohort studies may be able to approximate regional incidence estimates and can provide important data to identify, stratify, and direct care and resources for pregnancies at highest risk. Future sub-studies using data and stored biological specimens from the ZAPPS cohort will aim to identify underlying biological mechanisms, causal pathways, and appropriate interventions for the accurate prediction and prevention of adverse birth outcomes in Zambia and worldwide.
Open Science Framework: Zambian Preterm Birth Prevention Study (ZAPPS) – Outcomes. https://doi.org/10.17605/OSF.IO/WT6Q834
This project contains the following underlying data:
- Z1A minimum dataset 2019-06-30.csv (underlying data for all participants)
- Z1A Codebook 2019-06-30.rtf (codebook for the variables within the dataset)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors acknowledge the invaluable contributions to the study design and conduct by Eve Lackritz, James Litch, Marcela Castillo, Nancy Hancock, and Nurain Fuseini. The study protocol is registered at ClinicalTrials.gov, identifier: NCT02738892.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Maternal and child health
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Genetics of pregnancy outcomes
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 24 Jan 20 |
read | |
|
Version 1 04 Sep 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)