Keywords
Schistosomiasis, WHO guidelines, Elimination as a public health problem, Mass drug administration, NTD Modelling Consortium
This article is included in the 2030 goals for neglected tropical diseases collection.
Schistosomiasis, WHO guidelines, Elimination as a public health problem, Mass drug administration, NTD Modelling Consortium
Following helpful reviewer comments, we have revised our letter to improve the clarity of our insights. Specific points added are as follows: uncertainty around how reliable the current WHO definition of EPHP is for estimating a reduction in schistosomiasis-related morbidity; our modelling assumptions on treatment coverage and adherence; input from the Global Schistosomiasis Alliance meeting to show views of its members. More references have also been added where needed.
See the author's detailed response to the review by W. Evan Secor
See the author's detailed response to the review by Stefanie Knopp
See the author's detailed response to the review by Darin Evans
The views expressed in this article are those of the author(s). The opinions expressed herein are those of the authors and do not necessarily reflect the views of the World Health Organization. Publication in Gates Open Research does not imply endorsement by the Gates Foundation.
Schistosomiasis remains an endemic neglected tropical disease (NTD) affecting approximately 220 million people worldwide1. It is an intestinal or urogenital disease caused predominantly by Schistosoma mansoni or S. haematobium. Individuals become infected when cercariae, released by freshwater snails, penetrate the skin during contact with contaminated water2. The disease can result in anaemia, chronic pain, diarrhoea, and malnutrition, causing poor school performance and lower fitness3. Donations of the treatment drug, praziquantel, are typically offered in school-based or community-wide mass drug administration (MDA) programmes for schistosomiasis.
The World Health Organization (WHO) has set goals of morbidity control and elimination as a public health problem (EPHP) for schistosomiasis to be reached by 2020 and 2025, respectively4,5 (defined in Table 1). There are recommended WHO treatment guidelines for achieving these goals based on the prevalence in school-aged children (SAC; aged 5–14 years old) prior to treatment. In low prevalence settings (≤10% SAC prevalence prior to treatment), MDA once every three years is recommended; in moderate prevalence settings (10–50% SAC prevalence prior to treatment), MDA once every two years is recommended; and in high prevalence settings (≥50% SAC prevalence prior to treatment), annual MDA is recommended4,5. MDA coverage has mainly focused on reaching 75% of SAC with treatment of adults at risk also recommended4,5. The WHO end goal for schistosomiasis is interruption of transmission (IOT) which is achieved once the incidence of infection is reduced to zero4,5. In May 2019, following a Global Schistosomiasis Alliance consultation meeting with its members and the WHO, there was support for the IOT goal with an interim and complementary goal of reducing the burden of schistosomiasis6.
Mathematical models of transmission dynamics and the impact of control interventions have been developed to inform decision makers on the optimal treatment strategies which are required for achieving the WHO goals. The Gates-funded NTD Modelling Consortium brings together multiple institutional groups working on NTDs, including schistosomiasis. Modelling groups based at Imperial College London (ICL) and Case Western Reserve University (CWRU), along with other collaborators have led the recent work for schistosomiasis. A model comparison was carried out for the ICL and CWRU models, and a joint policy paper was also produced7,8. Due to knowledge gaps surrounding the epidemiology of schistosomiasis, the models have contrasting underlying assumptions leading to differences in model predictions8. Despite these differences, the models generally agree on the treatment strategies required to achieve EPHP for S. mansoni, thereby strengthening the evidence for our model recommendations7.
Moving towards the post-2020 goals, new WHO goals have been proposed for the NTDs to be reached by 2030. Currently, the proposed 2030 goal for schistosomiasis is EPHP. Using the insights that have been gained from recent modelling work on S. mansoni, we highlight the practical implications of EPHP (the timelines and feasibility of achieving EPHP) and the risks that need to be mitigated to maintain this goal. There is uncertainty around how reliable the current WHO definition of EPHP is for estimating a reduction in schistosomiasis-related morbidity as lower intensity infections may also be associated with significant morbidity3. Further modelling will be required following revision of this goal by WHO as this may impact our recommended treatment strategies.
Note that the following sections focus on S. mansoni and Kato-Katz (as this is the currently recommended diagnostic technique9). Additionally, the current WHO treatment guidelines and EPHP goal have been investigated here but these are currently under revision by WHO. Importantly, our modelling insights remain relevant as we highlight where the current guidelines are sufficient and where programmatic adaptations are needed for achieving the current EPHP goal (refer to Table 1 for a summary).
Using models developed independently by ICL and CWRU, we investigated whether the currently recommended WHO guidelines (of 75% SAC-only treatment) are sufficient for achieving the EPHP goal for S. mansoni. Our modelling and data analyses showed that these guidelines are sufficient for reaching EPHP in low to moderate settings7,10. However, as prevalence rises within high settings, an increase and expansion in treatment coverage to include adults, as well as SAC, is required to reach EPHP with coverage levels dependent on the setting7,10 (Table 2). As the burden of infection (intensity of transmission) in adults relative to SAC increases, the coverage levels needed to achieve EPHP increase (Figure 1)10. Coverage levels also increase if EPHP is to be achieved within a shorter amount of time (Figure 1).
SAC refers to school-aged children aged 5–14 years old.
| Prevalence in SAC prior to treatment | Model recommended treatment strategy for achieving EPHP |
|---|---|
| Low (<10%) | 75% SAC treatment once every 3 years within 6 years7. |
| Moderate (10%–50%) | 75% SAC treatment once every 2 years for up to 5 years (this holds for low to high adult burdens of infection)10. |
| High (≥50%) | As prevalence rises, SAC and adult annual treatment with coverage levels increasing with the adult burden of infection (coverage also increases as programme duration shortens; shown for 5–10 year programmes in Figure 1)10. |
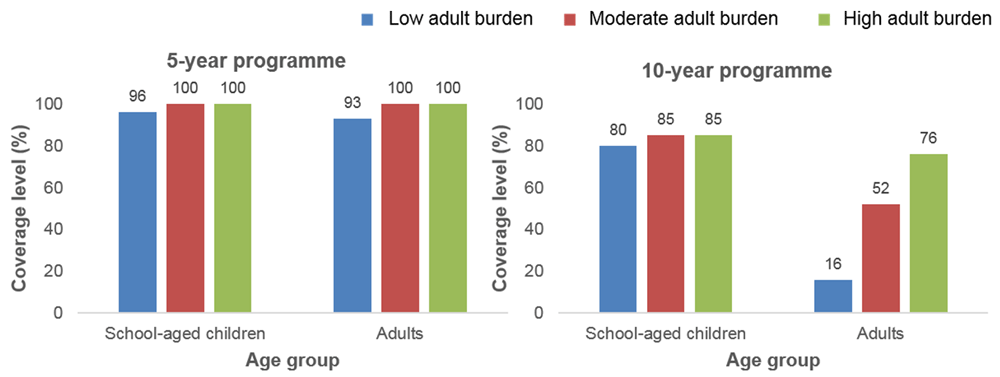
School-aged children (SAC) are 5–14 years old and adults are 15+ years old. This figure has been reproduced from 10 under a Creative Commons Attribution 4.0 International (CC BY 4.0) license.
Monitoring and evaluation (M&E) programmes are used to collect data to assess the progress of a treatment programme and to determine the appropriate treatment strategy. M&E data are typically collected from SAC as they are relatively easy to sample from. However, as the optimal treatment strategy for S. mansoni depends on the burden of infection in SAC and adults, M&E prevalence and infection intensity data need to be collected from a broader age-range10. Our work has also shown that despite achieving EPHP, the prevalence may still be high due to light- to moderate-intensity infections persisting in SAC, in addition to all the infections remaining in pre-SAC and adults7,10. Therefore, stopping treatment after reaching EPHP poses a high risk of resurgence.
The treatment strategy required to achieve EPHP is determined by the epidemiological and ecological setting, such as the baseline prevalence/transmission intensity7,10. EPHP is technically feasible in all settings within 10 years provided that the appropriate treatment strategy is used. Table 2 shows the model recommended treatment strategies. Achieving and maintaining high coverage, adherence and treatment opportunities over each round of treatment is essential11. Here, we have assumed treatment at random with full adherence at each round of MDA. Areas with poor school enrolment may benefit more from community-wide treatment12.
To monitor and assess progress towards the EPHP goal, prevalence and infection intensity data are required from SAC (as the goal is defined by <1% prevalence of heavy-intensity infections in SAC). The goal is typically assessed by averaging the prevalence measured in five schools randomly sampled within a district13. This approach does not take into account the high spatial heterogeneity and focality in Schistosoma prevalence. Taking implementation decisions at the district level using the currently proposed sampling strategy can lead to under- and over-treatment of SAC. Sampling fewer children in more schools has been shown to improve prevalence estimates, reducing under-treatment13. Ongoing work on mapping protocols will allow for more precise targeted treatment.
Kato-Katz is currently the recommended diagnostic test, but there are relatively newer, more sensitive diagnostics available. Due to the reduced sensitivity of diagnostic techniques at low prevalence levels, the true prevalence is likely to be higher than the measured prevalence. Prevalence measured with Kato-Katz will be lower relative to that measured with more sensitive diagnostics, such as point-of-care circulating cathodic antigen (POC-CCA) tests, and this difference has been analysed, although the relationship between the two diagnostics remains unclear14–16. Therefore, the diagnostic technique used will impact the sampling strategy, with a more sensitive diagnostic likely facilitating the sampling of fewer people or the use of higher prevalence thresholds when measuring EPHP and furthermore IOT17.
Accurate, representative data on which age groups are infected are required to determine the most cost-effective treatment strategy, for example, only collecting data on high-risk adults can overestimate the benefit of community-wide treatment12. The costs of diagnostic techniques also need to be considered. Although the traditional Kato-Katz diagnostic is seen as the cheaper test, given the increased sensitivity of POC-CCA, this may outweigh costs in the long term18.
There are risks that need to be mitigated to achieve EPHP. Individuals with no access to treatment or those not taking treatment in any round of MDA (systematic non-adherers) may result in maintained transmission11,19. Due to systematic non-adherence, reported coverage may be higher than true coverage19. Ideally data on adherence as well as coverage should be collected within M&E programmes as both will impact the outcome of treatment programmes19.
M&E programmes focus on SAC, and may be biased to those who are treated, making it difficult to promptly identify a failing treatment programme. Therefore, it is vital that the M&E data collected is representative of each age group10,12. Manipulation of implementation unit size may mask persistent prevalence of challenging locations, such as hotspots. Guidance on mapping of schistosomiasis prevalence will aid in determining the optimal size of implementation units. Further risks which may reduce the effectiveness of treatment programmes are potential drug resistance (declining praziquantel efficacy following multiple rounds of treatment20) and the presence of zoonotic reservoirs21,22. More insights are needed on such risks as more intensified treatment strategies than those currently recommended here may be required if they are present.
Following achievement of EPHP, infections may remain present in the population resulting in resurgence if treatment is stopped7,10. Pre-SAC can also be infected with schistosomes and a reservoir of infection may remain in this age group following MDA to other age groups. Development of a paediatric formulation of praziquantel for pre-SAC treatment would prevent this23. Due to remaining infections, it is highly likely that treatment will still be needed to maintain control after achieving EPHP24. Good water, sanitation and hygiene could aid in sustaining EPHP, allowing treatment to be scaled down25.
To alleviate the need for ongoing treatment and to prevent resurgence, IOT is required after reaching EPHP2,7,10. The transition of treatment programmes from EPHP to IOT will require reassessment of the treatment strategy, with consideration of complementary interventions such as behaviour change and snail control. Once very low prevalence levels have been achieved and a treatment programme is stopped, surveillance is needed to ensure that IOT has been achieved and that resurgence has not occurred. Currently, there is little guidance available for programmes when stopping treatment. Recently, the ICL model determined the post-treatment surveillance criteria for predicting IOT for S. mansoni. Results showed that a 1% Kato-Katz prevalence measured 2 years (or later) after stopping treatment across 200 individuals (randomly sampled from all age groups in a population of 500–1000 individuals), means IOT is 90% likely in the absence of re-introduction17.
| Priority issue / question identified in discussion with WHO | How can quantitative and mathematical modelling address this? |
|---|---|
| Re-run the models with the broad parameters of the new treatment guidelines. | New guidelines can be simulated in the model and followed through to determine if they are sufficient for achieving EPHP (as done previously for current guidelines7). |
| Quantitative assessment of morbidity averted with continued treatment. | Modelling can simulate new guidelines to determine heavy-intensity infection prevalence and overall prevalence cases averted which can be related to morbidity averted. |
| How do we know when country settings can transition from EPHP to IOT? • What interventions are required? • What criteria are required? • Where possible? • What are the cost implications? | Modelling has been used to show the MDA treatment strategy required to achieve EPHP7,10. This can be extended to investigate the interventions required for transitioning to IOT. Modelling can then investigate the feasibility of sustaining EPHP versus moving to IOT. IOT prediction and post-MDA surveillance criteria have been determined for S. mansoni17. |
No data are associated with this article.
Members of the NTD Modelling Consortium Schistosomiasis Group:
Jaspreet Toor (j.toor@imperial.ac.uk; corresponding author)1,2, Claudio Fronterre (c.fronterr@lancaster.ac.uk)3, Joaquin M Prada (j.prada@surrey.ac.uk)4, Charles H King (chk@case.edu)5, T. Déirdre Hollingsworth (deirdre.hollingsworth@bdi.ox.ac.uk)6, Graham F Medley (graham.medley@lshtm.ac.uk)7, Roy M Anderson (roy.anderson@imperial.ac.uk)1,2,8
1London Centre for Neglected Tropical Disease Research, Department of Infectious Disease Epidemiology, St Mary’s Campus, Imperial College London, Norfolk Place, London W2 1PG, UK.
2MRC Centre for Global Infectious Disease Analysis, Department of Infectious Disease Epidemiology, School of Public Health, Faculty of Medicine, St Mary’s Campus, Imperial College London, Norfolk Place, London W2 1PG, UK.
3Centre for Health Informatics, Computing and Statistics (CHICAS), Lancaster Medical School, Lancaster University, Lancaster LA1 4YW, UK.
4School of Veterinary Medicine, Faculty of Health and Medical Sciences, University of Surrey, Guildford, GU2 7AL, UK.
5Center for Global Health and Diseases and Department of Mathematics, Case Western Reserve University, 10900 Euclid Avenue LC: 4983, Cleveland, OH 44106, USA.
6Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Old Road Campus, Headington, Oxford, OX3 7LF, UK.
7Centre for Mathematical Modelling of Infectious Disease, London School of Hygiene and Tropical Medicine, 15-17 Tavistock Place, London, WC1H 9SH, UK.
8The DeWorm3 Project, The Natural History Museum of London, London SW7 5BD, UK.
We would like to thank Hugo C Turner, Marleen Werkman and James E Truscott for contributing to the work represented in this article. We thank Torey de Rozario, Simon Brooker and other members of the Gates Foundation NTD team for providing valuable feedback on this article. We also thank Maria-Gloria Basanez and Sake J de Vlas for providing helpful comments. Additionally, we are grateful to Andreia Vasconcelos for overlooking the development of this article.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Is the rationale for the Open Letter provided in sufficient detail?
Yes
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Yes
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Yes
References
1. Li E, Gurarie D, Lo N, Zhu X, et al.: Improving public health control of schistosomiasis with a modified WHO strategy: a model-based comparison study. The Lancet Global Health. 2019; 7 (10): e1414-e1422 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Preventive chemotherapy/mass drug administration, public health policy, schistosomiasis, onchocerciasis, STH, LF
Is the rationale for the Open Letter provided in sufficient detail?
Partly
Does the article adequately reference differing views and opinions?
Partly
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Partly
Is the Open Letter written in accessible language?
Partly
Where applicable, are recommendations and next steps explained clearly for others to follow?
Yes
References
1. Knopp S, Ame SM, Person B, Hattendorf J, et al.: A 5-Year intervention study on elimination of urogenital schistosomiasis in Zanzibar: Parasitological results of annual cross-sectional surveys.PLoS Negl Trop Dis. 2019; 13 (5): e0007268 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology and control of helminth infections; elimination research
Is the rationale for the Open Letter provided in sufficient detail?
Yes
Does the article adequately reference differing views and opinions?
Yes
Are all factual statements correct, and are statements and arguments made adequately supported by citations?
Partly
Is the Open Letter written in accessible language?
Yes
Where applicable, are recommendations and next steps explained clearly for others to follow?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 19 Nov 19 |
read | ||
|
Version 1 16 Aug 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)