Keywords
Differential Pricing, Access, Affordability, Hypertension, Diabetes, Control
Differential Pricing, Access, Affordability, Hypertension, Diabetes, Control
Low-and-middle income countries (LMICs) are experiencing an epidemiologic transition characterized by a dramatic rise in the burden of non-communicable diseases (NCDs)1–6. Hypertension and diabetes mellitus (DM), two of the principal risk factors for cardiovascular diseases (CVDs), are under recognized, untreated or under-treated in these regions resulting in considerable morbidity and mortality7. Many factors including low literacy rates, non-adherence, therapeutic inertia, and systemic factors such as challenges in access and affordability of medicines have been ascribed as reasons for poor disease control8–11.
Affordability of quality assured, innovator medicines for the management of hypertension and DM in resource-limited settings is challenged by low income10,12–14. As populations age and disease prevalence increases, innovative and cost-effective interventions are urgently needed to improve access to these medicines for sustained and life-long management of these conditions. Differential pricing (DP) of medicines is a promising and viable approach to improve access and affordability. DP is an approach by which manufacturers price their medicines to reflect patients’ ability to pay and has been successfully deployed to increase access to quality assured anti-malarials and vaccines for immunization15–18. However, such an approach has hitherto not been explored for the management of non-communicable diseases. To test DP as an intervention for improving access to innovator medicines for the control of hypertension and DM, the Bill and Melinda Gates Foundation working with three pharmaceutical companies made differentially priced medicines for these conditions for this study in Ghana. Our hypothesis is that by offering innovator medicines at a two-tier pricing system, i.e. market price and a lower tiered differential price based on socio-economic status, we could in a systematic fashion test whether a DP scheme improves access and affordability of medications. Furthermore, we sought to assess whether purchasing innovator medications was associated with better control of hypertension and DM.
The study protocol has been published previously19.
This study protocol was approved by the Committee on Human Research Publications and Ethics of the Kwame Nkrumah University of Science and Technology (CHRPE/AP/298/14) and the Ghana Health Services Ethical Review Committee (GHS-ERC: 12/07/14). It was declared exempt by the Institutional Review Board at the Johns Hopkins Bloomberg School of Medicine. Written informed consent was obtained from all study participants before enrollment into the study. All relevant data are included in the manuscript and as Underlying and Extended data.
The Ghana Access and Affordability Program (GAAP) study is a quasi-experimental study with a pragmatic trial design to examine the effect of DP on improving access to innovator medicines for control of hypertension and diabetes in a multi-center, prospective Ghanaian cohort.
The GAAP study was conducted at five hypertension and diabetes specialty and general clinics in urban, semi-urban and rural locations in Ghana. The study was conducted at two tertiary institutions - Komfo Anokye Teaching Hospital (KATH) and Tamale Teaching Hospital (TTH) - two secondary level health institutions - Agogo Presbyterian Hospital (APH) and Atua Government Hospital (AGH) - and one primary level health institution - Kings Medical Center (KMC).
Participants were eligible if they were ≥18 years with known diagnosis of hypertension and/or type II diabetes presenting for routine care at either a general polyclinic (AGH, KMC, TTH) or a dedicated diabetes/hypertension clinic (KATH, APH). During the period of the study, consecutive potential participants were invited to participate after study nurses had explained the objectives of study. Participants meeting eligibility criteria were enrolled after written informed consent had been obtained by trained research assistants.
At enrollment participants were assigned to DP by research assistants if their monthly household income was <210 Ghana Cedis (i.e. minimum wage) or had a multidimensional poverty index score ≥ 6/1820. Study participants whose physicians prescribed study medications were to purchase them at either DP or MP from the hospital pharmacy. However, when participants allocated to MP could not afford prescribed medicines at MP, they were offered pricing at the lower tiered DP at the pharmacy. Participants who could not purchase study medicines at either MP or DP were prescribed generic alternatives available on the National Health Insurance Scheme21. The decision tree is shown in Figure 1.
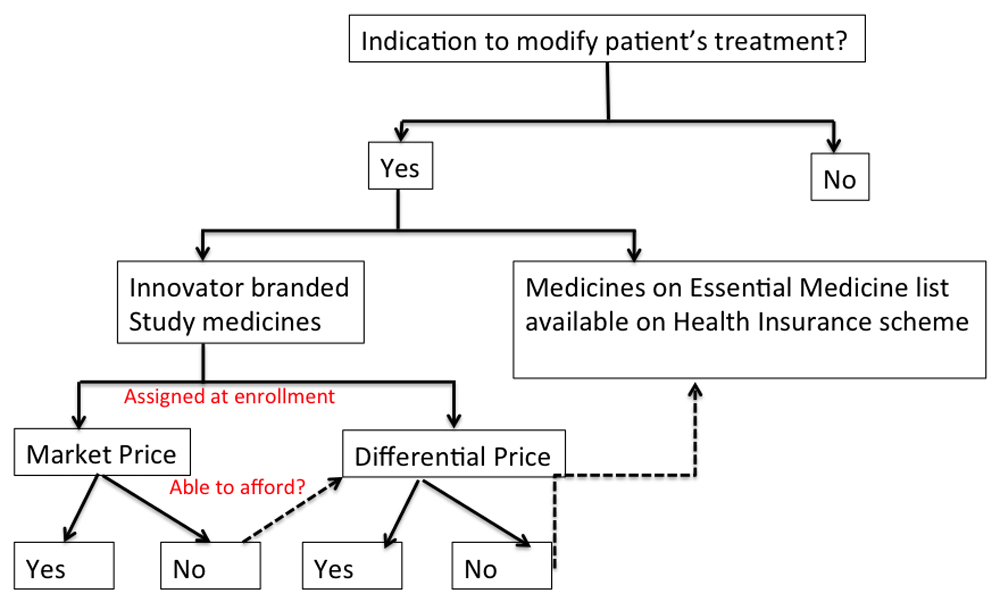
Physicians exercised their independent judgment in deciding on whether or not they would prescribe innovator brands of anti-hypertensive or anti-diabetic medicines at market price (MP), differential price (DP) or use generic alternatives where indicated. Participants prescribed innovator brands were asked to determine if they could purchase prescribed medicines within 2 days of prescription to avert leaving them uncontrolled for their medical condition. Participants assigned to MP but could not purchase prescribed medicines at MP, were offered the opportunity to purchase study medicines at DP. Participants who could not afford to purchase prescribed medicines at either MP or DP were offered generic alternatives of anti-hypertensive or anti-diabetic medications as indicated.
Trained Research Assistants interviewed participants and collected demographic and household information such as age, gender, educational attainment, employment status, monthly income and health expenditures. Study participants were also interviewed on their lifestyle behaviors such as alcohol use, diet, cigarette smoking and physical activity. We categorized alcohol intake and cigarette smoking status as never, former or current users. Physical activity was assessed by asking if participants frequently performed physical activities that caused a small increase in breathing or made their heart rate go up, such as (fast/brisk) walking, jogging, bicycling, and how much time they spent doing physical activity.
A detailed medical history including duration of hypertension or diabetes and medication used for treatment were obtained. Compliance to treatment was assessed using the 14-item Hill-Bone compliance to high blood pressure therapy scale22 and the 4-item version of Levine-Morisky Medication Adherence Scale23 among diabetes patients. A past medical history of stroke was elicited by asking if participant had ever experienced sudden onset of weakness or sensory loss on one side of the body, sudden loss of vision, or sudden loss of speech. Similarly, a history of heart failure was assessed by asking if participant had ever experienced shortness of breath on exertion, on lying down as well as swelling of both feet. Blood pressure and pulse rates were measured by study nurses following a standardized study protocol using an automated BP measurement device (Omron HEM-907XL). Two consecutive BP readings from the same arm taken 2 minutes apart was recorded and averaged for the present analysis. Anthropometric evaluations included measurements of weight and height for body mass index derivation and waist circumference.
To ensure standardization across all study sites, an ISO-certified, quality-assured laboratory in Ghana was contracted to run all biochemical panels including creatinine, lipid profile and hemoglobin A1C for participants with diabetes. Samples were transported to the laboratory on the day of collection often within 4 hours or where not feasible (KMC and AGH sites), samples were frozen and delivered to the laboratory the next day.
Following enrollment, each participant was seen at two monthly intervals by their physician for 18 months to assess disease control. At enrollment and during follow-up physicians used their clinical judgment to make any changes in medicines they felt, including adding or discontinuing study medications (either at MP or DP).
We developed a broad range of health systems strengthening activities, such as training of doctors on management of hypertension and diabetes using locally developed treatment guidelines (see supplementary material in 19) aligned with international guidelines, strengthening supply chain systems within health facilities to minimize important access barriers such as stock outs and medication expiry, and developing patient education leaflets on management of diabetes and hypertension19.
The following outcome variables were recorded:
Decisions by physicians to prescribe study medicines at clinic visit
Number of times out-of-pocket payments for study medications were made
Blood pressure control: systolic and diastolic blood pressure readings taken at 2 monthly visits were averaged for each participant.
Glycemic control: HbA1C measurements taken at 6-monthly were averaged with a target of <7.0%.
Means and medians were compared using either the Student’s t-test or Mann-Whitney’s U-test for paired comparisons or ANOVA or Kruskal Wallis tests for more than 2 group comparisons. Proportions were compared using the Chi-squared tests or Fisher’s exact test for proportions with subgroupings <5. A multivariate logistic regression analysis was performed to identify factors associated with ability to purchase study medications. Predictors selected in this analysis include age, gender, location of residence, educational status, employment status, monthly household income, price allocation (DP or MP), willingness to purchase study medications should they be prescribed and level of health facility. These factors were selected based on their known or predicted impact on ability to make out-of-pocket payments for medications. A parsimonious logistic regression model was constructed to determine the predictors of BP and glycemic control among participants who purchased study medicines compared with those who could not purchase prescribed study medicines. Variables included in these models included demographic variables, socio-economic indicators, level of healthcare institution, adherence to therapy and number of times purchases of innovator branded study medicines were made. In bivariate analysis, factors that attained a p-value of <0.05 were included in the multivariable model with two-tailed p-values <0.05 considered statistically significant. Statistical analysis was performed using SPSS version 19.
There were 3,296 participants enrolled into the study with a mean ± SD age of 57.5 ± 12.7 years and a preponderance of females comprising 76.6% of the study population. There were more urban dwellers (43.6%), followed by rural (33.7%) and semi-urban dwellers (22.6%). There were 1,869 (56.7%) participants with hypertension only, 422 (12.8%) with diabetes only and 1,005 (30.5%) with both hypertension and diabetes. Overall, mean ± SD duration of hypertension was 7.8 ± 7.3 years and DM was 9.4 ± 6.9 years, with 42.0% of participants with BP controlled and 29.8% with optimal glycemic control at enrollment into the study (Figure 2). Comparisons of baseline characteristics according to study site and pricing arm allocation are shown in Extended data: Tables S1 and S2.
At enrollment, all participants were on generic antihypertensive and anti-diabetic medicines available on National Health Insurance. A total of 24,632 patient clinic visits occurred over the course of the study with 2,469 (10.0%) decisions to modify treatment (Table 1). Average follow-up per participant was 14 months. There was a higher tendency for physicians to prescribe generic medicines for treatment modifications than with innovator medicines 76.2% vs 23.8% respectively (p<0.0001). Overall, 238 (45.2%) participants were able to afford prescribed study medicines while 288 (54.8%) were unable.
| Characteristic | |
| Number of subjects recruited | 3,296 |
| Mean ± SD duration of follow-up per subject (months) | 14.2 ± 5.9 |
| Total number of clinic visits | 24,632 |
| Number (%) of decisions taken by physicians to modify patient’s treatment at clinic visits# | 2,469 (10.0%) |
| Decisions to use generic equivalents available on National Health Insurance scheme | 1,882 (76.2%) |
| Decisions to use innovator branded medications | 587 (23.8%) |
| Number of patients prescribed innovator branded medicines | 526 |
| Number of patients able to purchase innovator branded medicines at least once | 238 (45.2%) |
| Number of patients who could not purchase innovator branded medicines | 288 (54.8%) |
| Total number of prescriptions of innovator branded medicines presented at study pharmacy (n=1,681) | 1,681 |
| Total number of prescriptions of innovator branded medicines presented at pharmacy but not purchased | 1,223 (72.8%) |
| Total number of prescriptions of innovator branded medicines presented at pharmacy and purchased | 458 (27.2%) |
| Price tier innovator branded medicines were purchased (n=458) | |
| Market Price | 179 (39.1%) |
| Differential Price | 279 (60.9%) |
Table 2 shows a comparison of baseline demographic, socio-economic and clinical characteristics of participants who purchased prescribed study medicines (n=238), those who could not purchase prescribed study medicines (n=288) and those not prescribed any study medicines (n=2,770). These participants were able to access medicines on the National Health Insurance Scheme list21. Study medicines were considered mainly for participants whose BP and/or glycemic indicators were not optimally controlled at baseline. Several other differences observed between these three groups with regards to demographic and socio-economic characteristics, healthcare expenditures, lifestyle behaviors and disease control are shown in Table 2. Factors associated with ability to afford study medicines with their adjusted OR (95% CI) include monthly household income >1,000 GHS, 2.90 (1.13 – 7.43) [1USD = 4.5 GHS]; allocation to MP arm at enrollment, 1.62 (1.06 – 2.47); willingness to purchase additional medicines for disease management, 2.59 (1.00-6.77); and tertiary level healthcare seekers, 0.29 (0.14-0.59), as shown in Table 3.
Of the 238 participants, 97 (40.8%) assigned to DP arm purchased study medicines at differential price while 141 (59.2%) assigned to MP arm purchased at the market price (n=91), but the remainder could only purchase at differential price (n=50) although they were assigned to MP arm. In total, 177 (74.4%) participants purchased innovator antihypertensive medicines and 61 (25.6%) bought anti-diabetic medicines. Overall, of the 458 study medicines purchases made by the 238 participants, 39.1% were at MP and 60.9% at the DP. However, the ability to afford study medicines were not sustained as 66% of participants could procure them just once (Extended data: Figure S1). The mean ± SD number of purchases/refills of innovator medicines among those assigned to MP arm who purchased at MP was 1.7 ± 1.4, those assigned MP arm but purchased at DP was 2.4 ± 2.2 and finally those assigned DP who purchased at DP (n=96) was 1.8 ± 1.4, p=0.03 (by ANOVA). Each medication purchase was made for two months of supply.
Among participants who purchased innovator antihypertensive medicines, systolic BP declined from a baseline 147.8 ± 21.7mmHg through to 138.3 ± 22.5mmHg at month 18, (p=0.0007 by ANOVA). Among those who could not purchase prescribed study medicines, systolic BP decreased from 149.5 ± 22.8 to 142.0 ± 26.6mmHg at month 18 (p=0.02, by ANOVA) (Figure 2). Similar trends were also observed in diastolic BP over time. Proportion of diabetics with HbA1C <7% increased from 23% at baseline to 39% at month 18 among participants who purchased study medicines for diabetes control, (p=0.10) and from 30% at baseline to 40% at month 18 (p=0.25) among those prescribed but could not purchase (Figure 3). In a parsimonious multivariate logistic regression model where age, gender, location of residence, duration of disease, income level and adherence were accounted for, purchase of antihypertensive medications for 6 or more times was associated with an adjusted OR of 4.09 (1.02-16.29) of achieving averaged BP<140/90mmHg during follow-up compared with those unable to purchase prescribed study medicines (Table 4). Similarly, purchase of study anti-diabetic medications for 5 or more times was associated with an adjusted OR of 6.73 (1.11-40.84) of achieving averaged HbA1c <7% (Table 5).
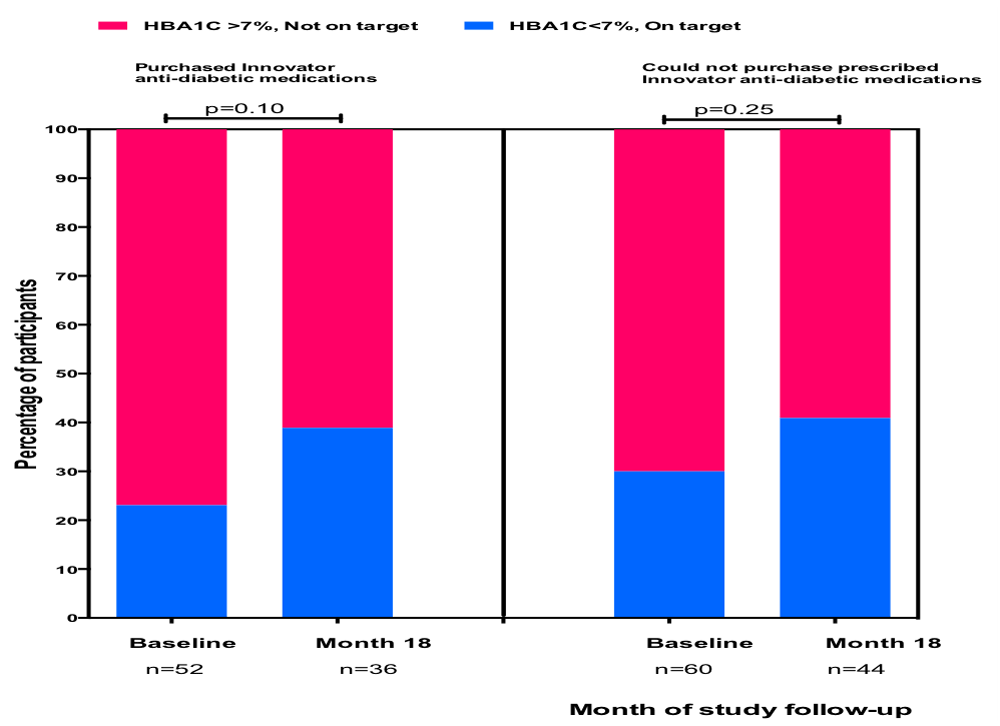
Comparison of proportion of participants with diabetes mellitus whose HbA1C <7% at baseline and at month 18 according to those who purchased innovator anti-diabetic medications and those who could not afford to purchase prescribed innovator medicines.
We have for the first time evaluated the effect of differential pricing on access to and affordability of innovator medicines for the control of hypertension and diabetes mellitus in a LMIC setting. Almost all study participants enrolled were already established on generic antihypertensive and or antidiabetic medicines. Hence study medications used to test the research hypothesis were prescribed when there was a therapeutic indication in accordance with physicians’ judgment. Modifications of existing medications were undertaken in approximately 10% of 24,632 clinic visits and physicians had a proclivity towards the use of generic branded medicines which were available on the Ghana National Health Insurance. Consequently, study medicines were prescribed for 526 (16%) study participants overall, of which about 45% were able to afford the prescription. However, <40% of study participants prescribed study medicines were able to purchase more than one prescription. Indeed, three fifths of all study medication purchases were at the lower DP tier for those assigned to DP arm and also for the significant majority who were assigned to higher tier MP arm but could only afford medications at DP. Hence, three out of the four factors independently associated with ability to purchase study medicines, namely MP arm allocation, willingness to make out-of-pocket payments and higher income levels, reflected higher purchasing ability of purchasers. However, the differential pricing intervention was intended to improve access for participants with lower income levels.
There are several possible explanations for the less than expected patronage of study medicines. First, the extent of reductions in prices of study medicines at the DP tier was not substantial enough to enhance its affordability in a resource-limited setting. The impact of price reductions by the participating pharmaceutical companies was also compromised by the various levies and mark-ups by distributors and hospitals resulting in a differential price for study medicines ranging between 20–40%, compared with the market price. Thus, the cost of study medications even at the differential price tier was beyond the means of many participants including those assigned to MP arm purported to have sufficient income levels to support out-of-pocket payments. In support of this, we found household income above 1,000 Ghana Cedis to be the only income bracket independently associated with ability to afford study medicines. Second, the Ghanaian National Health Insurance scheme covers the cost of most essential medicines for hypertension and diabetes for an annual premium of <USD524. There was a 98% subscription rate by study participants to this scheme. Thus, the proposition of making additional payments for medications was not popular. We found willingness to make out-of-pocket payments for study medicines as a factor associated with ability to purchase study medicines. Third, the range of study medicines used to test the study hypothesis had generic equivalents covered by National Health Insurance and might have obviated the need to prescribe them. Indeed, almost all study participants were already established on generic branded medications for hypertension and diabetes control at enrollment. For patients well controlled or not experiencing adverse reactions, physicians and patients may have not sensed a need for change to a costlier medicine. In spite of these challenges, we observed a trend towards improved control of hypertension and diabetes among the few participants able to afford study medicines on a more sustained basis.
It is estimated that approximately 90% of individuals in LMICs use their own funds to purchase medicines25. The out-of-pocket expenditures for medicines are second to food, making government subsidies for medicines not realistic25. Differential pricing may potentially contribute to attaining the sustainable development goal of Universal Health Care by assuring access to safe, effective, quality and affordable essential medicines26. However, as a strategy, differential pricing of essential medicines places the burden of medication purchases on the patient. This burden is dependent on the cost of medicines, the purchasing power of patients and the extent of price reduction on the product by the pharmaceutical company and the size of government taxes, levies, and mark-ups by distributors and health facilities. In LMICs purchasing power of the majority of patients living with NCDs requiring life-long treatment is low. Our findings would support the need to find additional ways to reduce prices of cardiovascular medicines for LMICs which currently bears the greatest burden of CVD on the globe27,28. Furthermore, in LMICs where national insurance policies are in existence, co-payment mechanisms for differentially priced innovator medicines to be shared between patients and national health insurance schemes would contribute to mitigating the financial burden on patients. Alternative price reduction mechanisms such as high-volume purchasing, reliable and adequate financing, public advocacy, negotiation, and market competition could contribute to further price reductions15–18. Further advantages could come through an integrated approach that addresses supply chain and weak health systems to improve access and affordability of quality assured innovator medications in LMICs. Governmental reduction or elimination of tariffs and charges on medicines together with hospitals dropping pharmacy mark-ups would also be helpful.
A major strength of this pragmatic study is the enrollment of participants at primary, secondary and tertiary health institutions situated in rural, semi-urban and urban settings to enhance generalizability of our findings. Our study is among the few from sub-Saharan Africa to prospectively evaluate BP and glycemic control and we found evidence of modest improvements in disease control (Figure 2 and Figure 3). Detailed analysis of determinants of disease control for the entire cohort is beyond the scope of the present report. We however exercise caution in over-interpretation of disease control rates in this prospective cohort in the light of high attrition rates during follow-up, which may have been influenced by survivorship bias. Also parsimonious logistic regression models were used to assess the impact of access to study medicines on disease control using a minimalistic set of covariates empirically known to be associated with disease control but not specified a priori.
Our findings have policy implications for pharmaceutical industry and governments. Strategic public-private partnerships and advocacy will be critical to the roll-out of differential pricing as a strategy to assure improved access and affordability of essential medicines for non-communicable diseases in LMICs. Health system strengthening activities such as regular and more frequent hospital visits as done in the present study, together with patient education and training of physicians at study sites may have contributed to different extents in the overall improvements in BP and glycemic control in this cohort. Undoubtedly, efforts at further improvements in hypertension and diabetes control are still urgently needed to avert the rising burden of CVDs and its accompanying unacceptably high morbidity and mortality from CVDs in this region29–40. Furthermore enhancing adherence to CVD medicines through mobile health technology as has been shown in the context of stroke in Ghana may be worth pursing further to prevent major adverse cardiovascular events from uncontrolled hypertension and diabetes mellitus41–43.
In conclusion, although purchases of study medicines were limited by cost even at differential price, sustained purchases of these quality assured medicines were associated with improved blood pressure and glycemic control. Further price reductions of study medicine below those in the present study are expected to lead to improved access and affordability of life saving cardiovascular preventive medications in LMICs.
Open Science Framework: Differential pricing of medicines to improve access to medicines for hypertension and diabetes control in Ghana: The Ghana Access and Affordability Program, a multi-center prospective trial, https://doi.org/10.17605/OSF.IO/C97HZ44.
This project contains the following underlying data:
Open Science Framework: Differential pricing of medicines to improve access to medicines for hypertension and diabetes control in Ghana: The Ghana Access and Affordability Program, a multi-center prospective trial, https://doi.org/10.17605/OSF.IO/C97HZ44.
This project contains the following extended data:
- Table S1: Baseline characteristics of study participants according to study site.
- Table S2: Baseline characteristics of study participants according to price allocation.
- Figure S1: Percentage of number of times innovator brands of medicines were purchased per participant.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Funding for this study was provided by MSD, Novartis, Pfizer, Sanofi (each a Participant Company) and the Bill and Melinda Gates Foundation [OPP1055800] (collectively, the Funders) through the New Venture Fund (NVF).
The NVF is a not-for-profit organization exempt as a public charity under section 501(c)(3) of the United States Internal Revenue Code of 1986 and assumes financial management of the study as a fiduciary agent and primary contractor for the Funders.
The participating pharmaceutical companies (Participant Companies) independently chose which innovator medicines to make available and the differential prices. Otherwise they had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit for publication. FSS had final responsibility for the decision to submit for publication.
Consistent with anti-trust laws that govern industry interactions, each Participant Company independently and voluntarily will continue to develop its own marketing and pricing strategies reflecting, among other factors, the Company’s product portfolios and the patients it serves. For the avoidance of doubt, the Participant Companies committed not to: (i) discuss any price or marketing strategy that may involve any Project-related product; or (ii) make any decision with respect to the presence, absence or withdrawal of any Participant Company in or from any therapeutic area; or (iii) discuss the launching, maintaining or withdrawing of any product in any market whatsoever. Each Participant Company is solely responsible for its own compliance with applicable anti-trust laws.
The Funders were kept apprised of progress in developing and implementing the study programs in Ghana but had no role in study design, data collection, data analysis or in study report writing.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Danzon PM, Towse A: Differential pricing for pharmaceuticals: reconciling access, R&D and patents.Int J Health Care Finance Econ. 2003; 3 (3): 183-205 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacoeconomics, pharmaceutical policy, and public health
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiovascular Prevention
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 07 Aug 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)