Keywords
Sustainability, water reuse, electrochemical disinfection, ultrafiltration, activated carbon, blackwater
This article is included in the Water, Sanitation & Hygiene gateway.
Sustainability, water reuse, electrochemical disinfection, ultrafiltration, activated carbon, blackwater
Electrochemical disinfection is a promising approach to sustainable decentralized waste water treatment because it enables oxidative inactivation of pathogens without requiring onsite storage of disinfecting chemicals (e.g., sodium hypochlorite or chlorine gas). In systems that utilize recycled blackwater for flushing, these processes become more energy intensive over time as solids accumulate in the process liquid1. Understanding how the constituents of blackwater that accumulate in such systems contribute to the decreased efficiency of electrochemical disinfection is key to developing remediation strategies that will enable practical implementation and long service lifetimes.
Previously, we investigated the effects on electrochemical disinfection energy efficiency of removing total suspended solids (TSS) with improved settling tank design2 and removing chemical oxygen demand (COD) with granular activated carbon (GAC)3 and found that only the latter resulted in a significant improvement. This implied that soluble COD was the principle cause of diminishing efficiency with repeated recycling of blackwater. However, because we had not completely removed TSS in any of these studies, we could not conclude definitively that suspended solids did not contribute. We also found that the same GAC media could remove a substantial fraction of blackwater COD in multiple treatment batches—suggesting that the filter medium was not fully saturated in these experiments—but that within each batch up to half of COD could not be readily removed by GAC3. Thus, we hypothesized that this poorly adsorbing fraction of COD was associated with suspended particulate matter not removed by settling or GAC, and further, that successful removal of this fraction from blackwater would improve the energy efficiency of subsequent electrochemical disinfection. We tested this hypothesis in a pilot study in which blackwater was treated by cross-flow ultrafiltration followed by a GAC packed bed filter, and assessed the effect of these combined pretreatments on the energy required for subsequent electrochemical disinfection.
Blackwater was collected from a prototype blackwater recycling toilet system previously described2. Procedures for the collection of human urine and feces used to generate blackwater were reviewed and approved by Duke University’s Institutional Review Board. Characteristics of the untreated blackwater used in this study are shown in Table 1.
Ultrafiltration was carried out in 8–12 L batches by passing blackwater through an ultra-high molecular weight polyethylene tubular membrane with a nominal pore size of 0.02 µm and a total active surface area of 0.07 m2 (Porex, Norcross, GA, USA) with a centrifugal pump (Lowara, Montecchio Maggiore, Italy) run in a recirculation configuration. In these experiments, flow was maintained between 28 and 30 L min-1 for a cross flow velocity in the retentate channel of 3.7 – 3.9 m s-1. Transmembrane pressures were monitored by pressure transducers (Omega PX039-015G5V, Omega, Norwalk, CT) on either side of the membrane connected to an Omega OM-DAQ-USB-2400 data logger, and during ultrafiltration typically ranged between 2 and 2.5 bar. Transmembrane flux was monitored by placing the permeate collecting vessel on a balance connected to a computer and using ADAM DU software to log changes in mass, and during ultrafiltration typically ranged between 80 and 120 kg m-2 h-1 (Figure S1).
Ultrafiltered blackwater was passed through a packed bed column filter with Aquacarb® 830, an 8 x 30 mesh-sized GAC derived from bituminous coal (Evoqua, Pittsburgh, PA), as the medium. The filter consisted of 1.8 kg GAC in a PVC pipe (9.4 cm inner diameter) with a media length of 58 cm, a media volume of ~ 4 L, and an interstitial volume of ~1 L. Liquid was pumped through at a rate of ~1 L min-1 in a recirculation configuration for up to 18 h. In a subset of experiments, COD was monitored during the first ~2 h of GAC treatment to evaluate the COD removal kinetics of GAC with ultrafiltered blackwater in comparison to untreated blackwater (Figure S2).
Electrochemical disinfection was performed as previously described3 in an 8-L HDPE tank with a commercially available electrochemical cell (Hayward Salt&Swim 3C) run at 24 VDC. Measurements of water quality parameters and microbial enumeration were performed as previously described in detail1–4. Energy required for disinfection was calculated as previously described1 and shown in Figure S3.
Statistical analyses and visualizations were performed using GraphPad Prism v7.04.
Results are presented in Figure 1. Ultrafiltration significantly reduced blackwater COD by an average of 55% (range 32–74%) and TSS by an average of 97% (range 92–100%). Subsequent treatment with GAC was associated with further reduction of COD to near or below the ISO 30500 category B standard (150 mg/L) for an average total COD reduction of 87% (range 75–99%). These reductions in COD and TSS were associated with a reduced energy demand for the electrochemical process to achieve the desired disinfection threshold (MPN < 5/ml) to an average of 8.5 kJ/L, which represents an order of magnitude improvement compared with the same process using untreated blackwater (70 kJ/L).
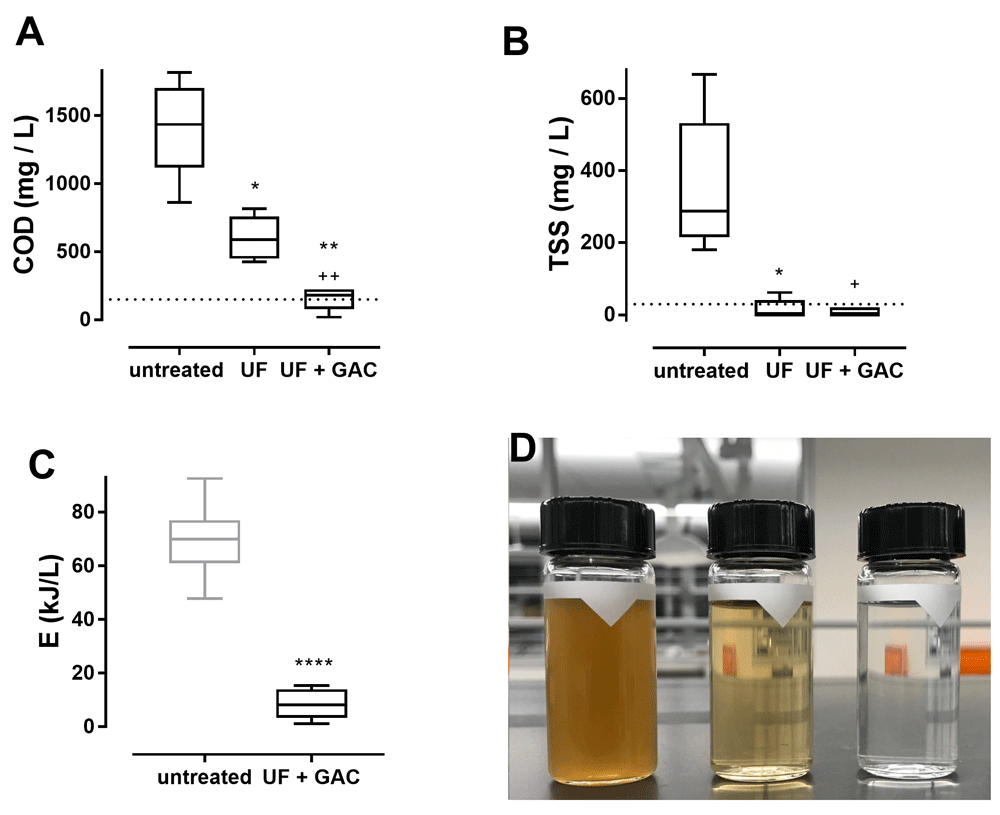
A and B: improvements in chemical oxygen demand (COD) and total suspended solids (TSS), respectively, with UF followed by GAC. Data are n = 5 batches, measurements taken sequentially in each batch. Dotted lines indicate ISO 30500 discharge standards (150 mg/L COD and 30 mg/L TSS). Significance determined by repeated measures one-way ANOVA with a Tukey’s multiple comparisons test. * = adjusted p < 0.05 vs. untreated, ** = adjusted p < 0.01 vs. untreated, + = adjusted p < 0.05 vs. UF, ++ = adjusted p < 0.01 vs. UF. C: Comparison of energy required to achieve disinfection (MPN < 5/ml) of blackwater treated by UF and GAC prior to electrochemical disinfection (n=5) to untreated blackwater (n=18). Untreated data are from a previous study3. Significance determined by two-tailed t-test, **** = p < 0.0001. A–C: lines indicate median, boxes 25th and 75th percentiles, error bars maximum and minimum values. D: Samples of untreated, UF treated, and UF + GAC treated blackwater (left to right).
Treating blackwater first with ultrafiltration, then with activated carbon, followed by electrochemical treatment, has specific advantages. The removal of suspended solids by ultrafiltration appears to allow for faster adsorption of soluble species by subsequent GAC treatment, which could make GAC treatment in a single-pass configuration practical and thus eliminate the need for a recirculating pump (Supplemental Data, Figure S2). Further, the removal of suspended solids minimizes the tendency of the GAC packed bed filter to clog, thus obviating the need for frequent backwashing.
As predicted, removal of both suspended and soluble COD reduced the energy required to achieve the desired disinfection threshold with the electrochemical process (Figure 1C). This lowered energy requirement allows for shorter runtimes on the electrochemical process, which will prolong the service lifetime of the electrodes. Furthermore, disinfection of pretreated blackwater is achieved with much shorter chlorine contact times compared to untreated blackwater, which means that system components (plumbing, tanks) in contact with the process liquid will have less exposure to oxidative chemistry and thus be less prone to degradation over time. Thus, though these results are preliminary, they point to an improved practical approach to onsite blackwater treatment.
Raw datasets are available on OSF, project “Improving energy efficiency of electrochemical blackwater disinfection through sequential reduction of suspended solids and chemical oxygen demand”, https://doi.org/10.17605/OSF.IO/GRMJT5.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
This study is supported by a Bill & Melinda Gates Foundation [OPP1164126].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figure S1: Ultrafiltration runs. Shown are plots of the transmembrane flux (in red) and transmembrane pressure (TMP, in blue) for each of the five ultrafiltration runs in this report.
Click here to access the data.
Figure S2: Effect of ultrafiltration of COD removal by GAC. Shown are plots of COD in one experiment with untreated blackwater and two with ultrafiltered blackwater. Blackwater was treated in 8-L batches by continuous recirculation at ~1 L min-1.
Click here to access the data.
Figure S3: Disinfection curves and energy calculations. Shown are plots of each electrochemical disinfection run following ultrafiltration and GAC treatment. Black dotted line indicates the disinfection threshold (MPN < 5/ml), red dotted line indicates the disinfection energy (Ed) interpolated from where the plot crosses the disinfection threshold.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental biotechnology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental engineering, biological treatment processes, applied microbiology, and molecular biology tools
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 23 Jan 19 |
||
|
Version 1 05 Oct 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)