Keywords
Tuberculosis, Diagnostic Test, HIV infection, South Africa
Tuberculosis, Diagnostic Test, HIV infection, South Africa
The manuscript has been revised in response to Reviewer comments regarding complicated presentation of the data and advice to exclude the LAM data. The major differences are 1) removal of the LAM data, 2) modification of Figure 1 to include informatoin regarding the basis for TB treatment and additional yield of Xpert, 3) modification of the supplementary file to include a figure 3 (new Figure 2 in supplementary material from the previous manuscript and clarification regarding the testing undertaken.
See the authors' detailed response to the review by Colleen F. Hanrahan
Since 2011 the World Health Organization (WHO) has recommended Xpert MTB/RIF (Xpert; Cepheid, Sunnyvale, CA) as the initial diagnostic test for individuals being investigated for HIV-associated tuberculosis (TB)1. TB diagnosis in people living with HIV (PLHIV) is complicated by the high proportion who are smear-negative and/or have extrapulmonary disease2. Although Xpert has superior sensitivity to sputum microscopy, it is less sensitive than culture, with a pooled sensitivity of 61% for smear-negative, culture-positive TB among PLHIV3.
South Africa replaced smear microscopy with Xpert starting in 2011, for all individuals with symptoms suggesting TB4. Further evaluation of those who are HIV-positive and Xpert-negative comprises clinical reassessment, chest radiograph if available, sputum for mycobacterial culture, and treatment with antibiotic if clinically indicated4. In a South African study of 394 patients investigated for TB (irrespective of presence of symptoms) prior to antiretroviral therapy (ART) initiation, the sensitivity of Xpert for smear-negative, culture-positive TB increased from 43% to 62% when a second sample collected at the first visit was tested5. Mathematical modelling using a decision model from South Africa6 suggested that replacing sputum culture with the cheaper option of a second Xpert would reduce loss to follow-up so 1% more patients would start TB treatment7, and save an estimated US$17.4 million per year7. This model assumed, based on limited data, the same sensitivity for the second Xpert test as for the first7, guidelines would be correctly followed7, and only 1% of those with TB symptoms start TB treatment based on a clinical diagnosis6. The strategy of sending a repeat Xpert for HIV-positive individuals whose initial Xpert result is negative has not been evaluated empirically.
The aim of our study was to describe the diagnostic yield from an immediate repeat Xpert on sputum, compared to sequential further investigation guided by South African recommendations, amongst HIV-positive adults being investigated for TB whose initial Xpert result is negative.
This “repeat Xpert” substudy was part of “Xpert for people attending HIV/AIDS care: test or review?” (XPHACTOR), a prospective cohort study evaluating a risk-based algorithm to prioritise Xpert testing amongst adults attending for routine HIV care in South Africa8.
XPHACTOR study flow, procedures and algorithm are described in detail in the supplementary material. In summary, we enrolled a systematic sample of adults (aged ≥18 years) attending four HIV clinics in Gauteng province, irrespective of presence of symptoms suggestive of TB, in the XPHACTOR study. Patients taking anti-tuberculosis treatment within the previous three months were excluded. Patients were enrolled into three groups: “on ART” (ART-experienced); “pre-ART” (in HIV care but not taking ART); and “HIV Testing and Counselling (HTC)” (newly-diagnosed HIV-positive). At the time of the study, ART eligibility comprised CD4 ≤350 cells/mm3 or WHO clinical stage ≥3. Research staff screened participants for TB at monthly intervals to three months, using a standardised questionnaire which incorporated the WHO symptom screen (any one of current self-reported cough, fever, weight loss or night sweats, hereafter the WHO tool). A spot sputum sample was collected for Xpert for individuals at a priori highest risk of active TB according to the study algorithm, which prioritised testing for those with any of: current cough, fever ≥ 3 weeks, night sweats ≥ 4 weeks, BMI <18.5 kg/m2, CD4 <100 cells/mm3, or weight loss ≥10%; and at enrolment from all in HTC group or pre-ART with CD4 <200 cells/mm3 (Supplementary material, Figure 1).
At enrolment and follow-up visits, participants who submitted an Xpert sample were reviewed within one week, and if Xpert-positive, TB treatment was initiated. If Xpert was negative, research staff repeated WHO symptom screen and facilitated the Xpert-negative algorithm for all who were WHO tool positive, which comprised chest radiograph, spot sputum for TB culture, and/or antibiotic trial as clinically appropriate. The Xpert-negative algorithm was also facilitated, because of a priori high risk of active TB, for all pre-ART participants with CD4<200x106/l who submitted sputum for immediate Xpert at enrolment to XPHACTOR.
At the three-month visit all participants had sputum and blood cultured for mycobacteria (Bactec MGIT 960 and 9240 systems). We allowed a broad window period around the three-month XPHACTOR main study final visit, until around six months, to maximise follow-up.
Study investigation results were returned to clinic staff, who were responsible for management decisions. Clinic records were reviewed at the end of the study to ascertain any additional relevant investigations (e.g. Xpert, mycobacterial culture and imaging requested as part of routine clinical care) and/or TB diagnoses.
XPHACTOR participants who were Xpert-negative with i) CD4 count<200 cells/mm3, or ii) new HIV diagnosis (HTC group) were eligible for this substudy, irrespective of presence of WHO tool symptoms; these restrictions aimed to minimise unnecessary testing of individuals at lower risk of active TB. If a participant had more than one negative Xpert result during follow-up, only the first episode was included.
At attendance for Xpert result review, eligible participants were asked for an additional spot sputum sample for “repeat” Xpert, which was frozen at -80°C within 24 hours of collection. All stored samples were thawed and tested with Xpert at the end of the study to evaluate the diagnostic yield that could have been achieved if an immediate repeat Xpert had been sent at the Xpert result review visit. We decided a priori not to induce sputum for this substudy in order to reflect what would be achievable in routine practice.
Repeat Xpert substudy entry and exit dates. Repeat Xpert substudy cohort entry date was defined as the date that the Xpert result review was conducted and sputum was collected for storage. Cohort exit date was defined as the last XPHACTOR study visit date.
TB case definitions. “Confirmed” TB was defined as a positive result on i) Xpert (on sputum sample) or ii) line probe assay (LPA) performed on smear-positive or cultured isolate (GenoType MTBDR plus, Hain Lifesciences) or iii) Mycobacterium tuberculosis (Mtb) culture, from any sample (including stored sputum and those requested by health care providers) collected within six months of XPHACTOR enrolment. Clinical TB was defined as TB treatment started within six months of enrolment ascertained from clinical records, self or family report, or reported in the context of a separate verbal autopsy sub-study, in the absence of microbiological confirmation. Six months was chosen because TB disease evolves gradually9,10; data from Zimbabwe estimated the mean duration of smear-positivity prior to TB diagnosis amongst HIV-positive adults at 18–33 weeks11.
“Not TB” was defined as absence of criteria for confirmed or clinical TB, and alive at least 3 months (the minimum follow-up period) after enrolment. Participants who did not fulfil the case definitions for TB or “not TB” were deemed to have unclassifiable outcome and excluded from analyses.
Pulmonary and extrapulmonary TB were classified in accordance with WHO definitions12.
We also summarise the basis on which treatment was initiated for those who started TB treatment.
Radiological definitions. “Probable radiological TB” was defined as presence of any of cavitation, predominantly upper lobe infiltrates, pleural or pericardial effusion, or clear miliary picture on chest radiograph. “Possible radiological TB” was defined as presence of any of lymphadenopathy (hilar or mediastinal), pulmonary nodules or other infiltrates. Participants with “probable” or “possible” radiological TB features, but without bacteriological confirmation, who started TB treatment within six months of substudy enrolment were assigned as having “clinical” TB.
The co-primary outcomes were a) the proportion of participants with a positive repeat Xpert and b) the proportion of participants fulfilling study TB case definitions.
Data were analysed using Stata 14 (Stata Corporation, College Station, TX, USA).
We did not undertake formal sample size calculation for this substudy as the sample size was all those eligible from the parent study.
We compared TB diagnoses made by Xpert using the sample stored at substudy enrolment, with all TB diagnoses fulfilling our case definitions during follow-up. We chose this pragmatic comparison because in real life, individuals with smear or Xpert-negative TB have sequential investigation, rather than all tests performed simultaneously. Our research staff facilitated the Xpert-negative algorithm when participants attended for Xpert result review, and therefore investigations are likely to have been initiated faster than in a routine setting. The proportion of TB diagnoses made by Xpert using the stored sputum was compared with TB diagnoses made during follow-up using McNemar’s test.
In a sensitivity analysis restricted to participants who had at least one component of the Xpert-negative algorithm (chest radiograph, sputum for TB culture, or antibiotic trial) within a two-week window of providing the stored repeat Xpert sample, we compared the proportion of TB diagnoses made by Xpert using the stored sputum with TB diagnoses made by the Xpert-negative algorithm using McNemar’s test.
The study was approved by the ethics committees at the University of the Witwatersrand (approval # M120343), University of Cape Town (approval # 106/2012), and the London School of Hygiene & Tropical Medicine (approval # 6165). All consenting participants gave written consent or, witnessed verbal consent if unable to read or write. All ethics committees approved the consent form. Principles expressed in the Declaration of Helsinki were followed in the conduct of this research.
Between September 2012 and March 2014, 235/410 (57.3%) potentially eligible participants were able to provide a sputum sample, stored for testing at study completion with Xpert (Figure 1). Eight participants with “unclassifiable” outcome were excluded, leaving 227 participants for analysis.
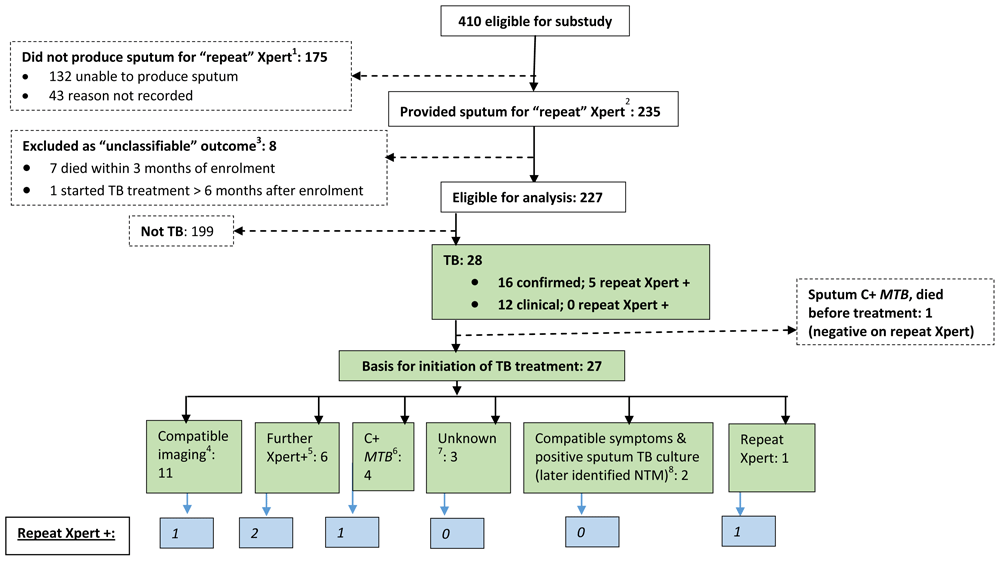
C+ = culture-positive; CXR = chest radiograph; MTB = mycobacterium tuberculosis; NTM = non-tuberculous mycobacteria; USS = ultrasound scan; Xpert+ = sputum Xpert MTB/RIF positive. 1 15/175 who were excluded because they did not produce sputum fulfilled case definitions for TB (clinical TB [9/15], confirmed TB [6/15]) and a further 3/175 had unclassifiable outcome 2 For 22/235 participants who provided more than one “repeat” sample (all Xpert-negative) only the result of the first sample and data from the associated review visit were used in the analysis 3 All had negative “repeat” Xpert. 4 CXR features compatible with “Probable TB” (pleural effusion [4], cavitation and infiltrates [2], miliary TB [1] who had positive repeat Xpert); CXR features compatible with “Possible TB” (2); USS features compatible with TB (Pericardial effusion [1]; abdominal TB [1]). 5 Xpert undertaken during study follow-up as part of routine clinical care or for research purposes in accordance with XPHACTOR study algorithm (Supplementary material, Figure 1). 6 Samples collected during study follow-up as part of routine clinical care or for research purposes (sputum Mtb culture-positive [3] of whom one had positive repeat Xpert, blood Mtb culture-positive [1]). 7 Two identified as having started TB treatment at verbal autopsy. One had M. xenopi identified in sputum culture prior to commencement of empiric TB treatment. 8 Started on basis of compatible symptoms and positive sputum culture later identified as M. avium (1) and M. intracellulare (1); both had improvement in symptoms after treatment was initiated.
Characteristics of the 227 substudy participants and comparison with the 175 excluded because they were unable to produce sputum are presented in Table 1. The majority of participants were female (63%), median age was 37 years (interquartile range [IQR] 31,44), median CD4 count was 100 cells/mm3 (IQR 51,147), and 26% had previously been treated for TB. 78/227 (34%) of participants reported a TB symptom, most often cough (23%, 52/227) or weight loss (19%, 43/227) (Table 1). Amongst the remaining 149/227 (66%) of participants who reported no WHO-tool symptoms at attendance for Xpert result, sputum was collected for repeat Xpert due to a priori high risk of active TB because newly-diagnosed HIV-positive (42); pre-ART with CD4 count <200 cells/mm3 (42); CD4 count <100 cells/mm3 (33); on ART with CD4 count 100–199 cells/mm3 (17); BMI <18.5 kg/m2 or weight loss ≥10%, (15). Enrolment to the repeat Xpert study was at median 7 days (IQR 7,8) from collection of the initial sputum sample for Xpert.
| Characteristic | Study participants (N=227) | Did not provide sputum for “repeat” Xpert (N=175) |
|---|---|---|
| N (%) | N (%) | |
| Demographics | ||
| Age, years - Median (IQR) | 37 (31-44) (N=226) | 36 (30-43) |
| Female | 144 (63.4%) | 109 (62.3%) |
| Black African | 222 (97.8%) | 175 (100%) |
| Participant category | ||
| On ART | 99 (43.6%) | 67 (38.3%) |
| Pre-ART | 75 (33.0%) | 60 (34.3%) |
| HTC | 53 (23.4%) | 48 (27.4%) |
| HIV/TB history | ||
| Previous TB treatment | 59 (26.0%) | 37 (21.1%) |
| Ever had IPT | 18 (7.9%) | 6 (3.4%) |
| Ever had CPT | 122 (53.7%) | 84 (48.0%) |
| BMI/CD4 when immediate Xpert was requested | ||
| BMI, kg/m2 - Median (IQR) | 23.3 (20.1-27.4) (N=226) | 23.4 (20.1-28.1) |
| CD41, cells/mm4 - Median (IQR) | 100 (51-147) (N=188) | 113 (56-169) (N=148) |
| WHO tool symptoms when sample for “repeat” Xpert was requested | ||
| WHO-positive | 78 (34.4%) | 44 (25.1%) |
| Cough | 52 (22.9%) | 23 (13.1%) |
| Weight loss | 43 (18.9%) | 32 (18.3%) |
| Night sweats | 16 (7.0%) | 9 (5.1%) |
| Fever | 7 (3.1%) | 2 (1.1%) |
| TB diagnoses over 6 months follow-up | ||
| Total | 28 (12.3%) | 18 (10.3%) |
| Confirmed TB | 16 (7.1%) | 7 (4.0%) |
| Clinical TB | 12 (5.3%) | 11 (6.3%) |
| Follow-up | ||
| Time from XPHACTOR enrolment to 3-month” study visit, days - Median (IQR) | 84 (84,95) (N=220) | 86 (84,106) (N=169) |
IPT= Isoniazid preventive therapy; BMI = body mass index; CPT= Cotrimoxazole preventive therapy; HTC= Enrolled from HIV testing and counselling service; WHO positive = self-report of any of current cough, fever, night sweats or unintentional weight loss.
1 Most recent clinic CD4 cell count when participant attended for Xpert result review. CD4 available for 188/227 participants enrolled (99/99 on ART, 75/75 pre-ART, 14/54 HTC); and 148/175 who did not provide sputum for repeat Xpert” (67/67 on ART, 60/60 pre-ART, 21/48 HTC)
12% (28/227) of substudy participants fulfilled case definitions for TB, of which 16 were confirmed and 12 were clinical (Table 2). One participant died before TB treatment could be commenced, and for one, the treatment start date was unknown. The remaining 26 started TB treatment at a median 49 days (IQR 0,108) after substudy entry. The range for time from substudy entry to earliest of positive TB investigation (including chest radiograph) or date TB treatment was started (amongst all fulfilling our case definitions for TB) was 0–118 days. 24% (19/78) participants who were WHO tool positive when the sample for stored repeat Xpert was collected fulfilled TB case definitions (confirmed 11, clinical 8).
| Characteristic | Participants diagnosed with TB N=28 N (%) |
|---|---|
| Case definition | |
| Bacteriologically confirmed TB: | 16 (57%) |
| Sputum Xpert positive1 | 6 (21%) |
| Sputum Mtbculture positive | 4 (14%) |
| Sputum both Xpert and Mtb culture-positive | 4 (14%) |
| Blood Mtb culture-positive | 1 (4%) |
| Pleural fluid cultured isolate LPA-positive | 1 (4%) |
| Clinical TB: | 12 (43%) |
| Site of TB | |
| Pulmonary TB only | 18 (64%) |
| Extrapulmonary TB only2 | 5 (18%) |
| Both pulmonary and extrapulmonary TB3 | 2 (7%) |
| Not recorded | 3 (11%) |
LPA = line probe assay
1 Includes two participants for whom bacteriological confirmation was provided by stored sputum which was Xpert-positive; one of whom started treatment based on this result, and the other had already started TB treatment because of compatible chest radiograph (miliary TB). 2 Pleural effusion (3); positive mycobacterial blood culture (1); pericardial effusion (1) 3 Compatible abdominal ultrasound and sputum Mtb culture-positive (1), pleural effusion and sputum Xpert positive (1)
Basis for commencement of TB treatment (n=27). Thirteen participants started treatment based on positive mycobacteriology. Amongst these, eleven started treatment because of a bacteriologically-confirmed TB result (Xpert [7, of which one was the stored repeat Xpert sample]; Mtb isolated from sputum [3] or blood [1]). A further two participants started treatment because of compatible symptoms and positive sputum mycobacterial culture (later identified as non-tuberculous mycobacteria [NTM]) with symptomatic improvement on standard TB treatment (Figure 1).
Eleven participants started TB treatment because of compatible imaging. Nine had compatible chest radiographs, of whom four were subsequently bacteriologically confirmed (Xpert 2, pleural fluid cultured isolate LPA-positive 1, positive Mtb sputum culture 1). Two participants started treatment based on ultrasound scans, one compatible with abdominal TB (subsequently confirmed by positive Mtb from sputum culture); and the other showing pericardial effusion. The basis for starting TB treatment was not clear for the remaining three participants (Figure 1).
Diagnoses made by repeat Xpert on stored sputum samples. 5/227 (2.2%) had a positive repeat Xpert. The sensitivity of repeat Xpert was 31.3% (5/16; 95% CI 11.0-58.7%) for the primary outcome of confirmed TB and 18% (5/28, 95% CI: 6.1%-36.9%) for the secondary outcome of confirmed / clinical TB combined) (Figure 2). In a matched analysis the odds of TB diagnosis was much greater by other modalities during follow-up than by the repeat Xpert, odds ratio 24.0 (95% CI: 3.9–986.9; p<0.0001, McNemar’s test). Amongst the five participants with positive repeat Xpert, three were in the pre-ART group, and two in the on ART group. We were unable to undertake multivariable analysis to look at independent predictors of positive repeat Xpert on the stored sample because only five were positive.
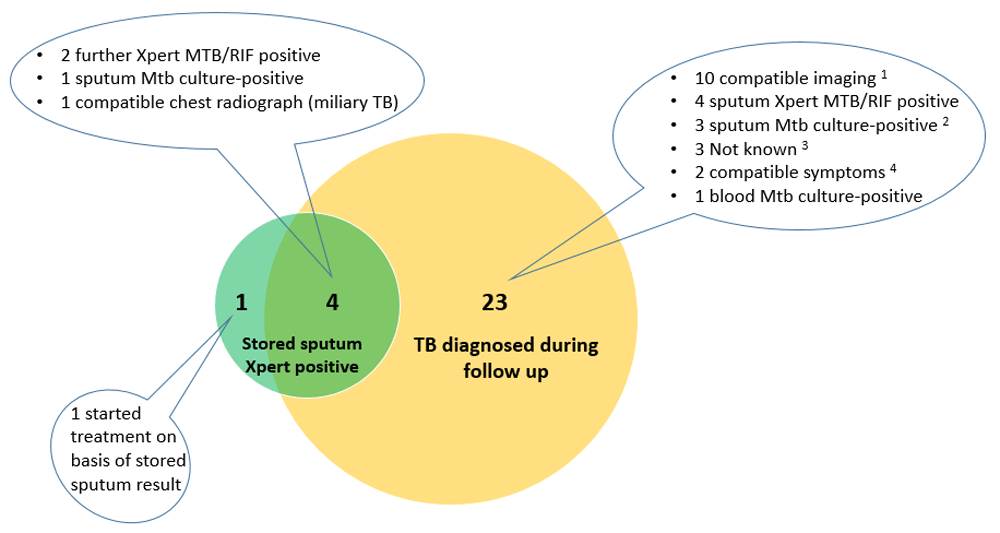
CXR = chest radiograph; USS = ultrasound scan 1 CXR features compatible with “Probable TB” (pleural effusion [4], cavitation and infiltrates [2]); CXR features compatible with “Possible TB” (2); USS features compatible with TB (Pericardial effusion [1]; abdominal TB [1]) 2 One participant died before treatment commenced 3 Two identified as having started TB treatment at verbal autopsy. One had M. xenopi identified in sputum culture prior to commencement of empiric TB treatment. 4 Started on basis of compatible symptoms and positive sputum culture later identified as M. avium (1) and M. intracellulare (1); both had improvement in symptoms after treatment was initiated.
In a sensitivity analysis restricted to 123 participants who had at least one component of the Xpert-negative algorithm within a two-week window of providing the stored repeat Xpert sample, 23 participants fulfilled our TB case definitions (13/23 confirmed, 10/23 clinical). The stored sputum sample was positive by Xpert for four participants (sensitivity of repeat Xpert for confirmed and clinical TB combined 17% [4/23]; for sputum culture-confirmed TB 20% [1/5]). Ten participants started TB treatment because of evaluation by the Xpert-negative algorithm (four confirmed, six clinical), of whom two also had positive stored repeat Xpert. Eleven other participants fulfilled TB case definitions during study follow-up (eight confirmed, three clinical). We did a matched analysis, classifying as “not TB” for the purpose of this analysis, 11 participants who fulfilled our TB case definitions but were not identified by either the Xpert-negative algorithm or stored repeat Xpert. The odds of TB diagnosis by the Xpert-negative algorithm was greater than by repeat Xpert, but did not attain statistical significance, odds ratio 4.0 (95% CI: 0.8–38.7; p=0.11, McNemar’s test).
The participant who started TB treatment solely based on the stored repeat Xpert sample was in the pre-ART group with a CD4 cell count of 113 cells/mm3 at substudy enrolment, had no previous history of TB treatment, and had a five week history of cough and fever when the initial sputum sample for Xpert was collected. The sputum culture, the only component of the Xpert-negative algorithm arranged at the Xpert review visit, was contaminated. This participant initiated ART on the day of entry to the substudy, was WHO-tool negative at all subsequent study visits, and had negative sputum and blood for mycobacterial culture at the 3-month visit. The remaining four participants with positive stored repeat Xpert sample started TB treatment before the stored sample was processed, based on further evaluation during follow-up: sputum Xpert-positive (2, one with rifampicin resistance); sputum Mtb culture-positive (1); and compatible chest radiograph (1).
Figure 2 in the supplementary material summarises all investigations undertaken for TB during substudy follow-up, aside from 3-month visit mycobacterial cultures and Xpert on stored sputum samples. As part of routine care or facilitated by research staff for the Xpert-negative algorithm, 97/227 (43%) had a chest radiograph (38/97 [39%] fulfilled criteria for radiological TB), and 100/227 (44%) had mycobacterial culture on sputum (3/100 [3%] Mtb positive). 34/227 (15%) of participants were prescribed an antibiotic trial at the Xpert result review, and 14/21 (67%) of those reviewed reported resolution of symptoms.
89 participants submitted sputum specimens for Xpert as part of routine care or because they fulfilled XPHACTOR algorithm criteria at monthly follow-up visits, for whom 6/89 (7%) were positive. An additional four participants had positive Xpert, of which three were stored sputum samples for repeat Xpert (bacterial confirmation provided solely by stored sample [2], also positive Mtb sputum culture [1]), and one was collected after the 3-month visit (Table 2).
The mycobacterial cultures performed routinely at the 3-month visit yielded Mtb isolates in 2% (5/219) of sputum and 0/220 blood samples.
Among HIV-positive individuals at high risk of active TB, with a negative sputum Xpert result, very few TB diagnoses would have been made in this study by immediately repeating Xpert. We limited our study to HIV-positive individuals at highest risk of active TB, i.e. those who were WHO tool positive with CD4<200x106/l, or pre-ART with CD4<200x106/l, or newly diagnosed, of whom some were asymptomatic, to minimise unnecessary testing of individuals at lower risk of active TB. The low yield from repeat Xpert in those with negative initial Xpert is likely due to paucibacillary or subclinical disease which is more likely in asymptomatic individuals, or extrapulmonary disease. These TB diagnoses may be better identified by alternative diagnostic modalities, such as chest radiography. A South African study of patients with sputum screened for TB by Xpert and mycobacterial culture prior to ART initiation, using a gold standard of culture-confirmed TB (N=85), found that those who were Xpert-negative had higher CD4 cell counts and lower viral loads than those who were Xpert-positive13. We did not have enough positive stored Xpert results to undertake a similar analysis.
Our study illustrates the realities of implementing the test negative algorithm in HIV-positive individuals. Despite research staff facilitating the algorithm, less than half (100/227) of participants produced sputum for mycobacterial culture during follow-up (vs. 100% assumed by Schnippel)7. We found sensitivity of the repeat Xpert was only 18% (5/28) for all TB or 31% (5/16) for bacteriologically-confirmed TB vs. 79% assumed by Schnippel6,7. Data from South Africa demonstrate poor adherence in routine care settings to TB diagnostic algorithms amongst HIV-positive individuals with initial negative Xpert test14,15. The aforementioned model6,7 assumes 1% of patients with TB symptoms start TB treatment based on a clinical diagnosis, but we found this to be far greater; and the model does not consider extrapulmonary TB (one-fifth of our participants diagnosed with TB had only extrapulmonary disease). An economic evaluation of repeat sputum Xpert vs. the Xpert-negative algorithm for HIV-positive individuals using assumptions that are more realistic is needed.
Evaluation of the 2007 WHO algorithm for smear-negative TB (comprising chest radiograph, single sputum for mycobacterial culture, and antibiotic trial), in HIV-positive individuals being investigated for TB in Cambodia16, against a gold standard of culture-confirmed TB based on multiple specimens, demonstrated sensitivity of 60%16. Sensitivity of this algorithm is imperfect, and there is a risk of overtreatment when only clinical-radiological features are used to start TB treatment. 40% (11/27) of our study participants who started TB treatment did so because of compatible imaging, of whom almost half were subsequently bacteriologically confirmed, highlighting its value to support rapid initiation of TB treatment. Our findings are in accord with data from the XTEND trial, which found that compatible chest radiograph was the main reason for initiating empiric TB treatment in a cohort of patients investigated for TB in primary care in South Africa, amongst whom microbiological confirmation was subsequently obtained for 13%17. South African national guidelines now recommend chest radiography for all individuals with symptoms suggestive of TB who cannot produce a sputum sample, but limited access to radiography facilities may limit implementation4. Amongst our study participants who provided sputum for mycobacterial culture prior to their 3-month visit there was a low yield of Mtb (3/100 [3%]), and the yield from further Xpert during follow-up was 7% (6/89), representing just over half (9/16) of all confirmed TB diagnoses. Our findings highlight the need for more sensitive diagnostic tests, and for repeating TB investigation using all available modalities, in HIV-positive individuals with initial negative sputum test result who remain symptomatic or have advanced immunosuppression.
Our study has some limitations. In the parent XPHACTOR study, Xpert testing was prioritised in people with BMI<18.5kg/m2 or CD4<100, those newly diagnosed HIV-positive or pre-ART with CD4<200, as well as those with TB symptoms8. Thus the population in this substudy did not all have classic “TB symptoms” at the time of collection of either initial or repeat sputum samples for testing with Xpert. These individuals may have had paucibacillary or subclinical disease which would not have been detected by Xpert. However, TB prevalence in our substudy population was high, and we anticipate our results to be relevant at least to these high-risk groups. Our results are also relevant to efforts to improve TB case finding among asymptomatic HIV-positive people18,19. We froze all our raw sputum samples within 24 hours of collection, and all were thawed and tested within 6 months of collection, in line with other studies20. We assumed that all participants starting TB treatment or with a sample which was bacteriologically confirmed collected within six months of enrolment were likely to have had active TB at enrolment, regardless of whether it was diagnosable using sputum based tests at the time of enrolment. In fact, our study participants who started TB treatment commenced within a median of seven weeks from collection of the “repeat” Xpert sample. Some sputum samples for mycobacterial culture and chest radiographs were taken at an interval after participants returned for their initial Xpert test result, reflecting real-life investigation practice; we cannot be certain of the same result if they had been performed at the same time as sample collection for repeat Xpert. However, our findings suggest that following the Xpert-negative algorithm is more likely to lead to TB diagnosis than immediate repeat Xpert test.
Strengths of our study include systematic evaluation of participants and longitudinal follow-up which minimised the number of TB diagnoses missed, and the pragmatic nature of the study which reflected as far as possible real-life conditions, albeit with optimised implementation of TB diagnostic algorithms.
Amongst ambulatory HIV-positive individuals at high risk of active TB, if an initial Xpert is negative, the Xpert-negative pathway should be implemented and there should be a low threshold for investigating those who remain at high risk using all clinically appropriate diagnostic modalities. In addition, those for whom no TB diagnosis is made must be made aware of the importance of returning for review if symptoms persist or recur. Our findings do not support sending an immediate repeat Xpert and highlight the need for more sensitive diagnostic tests capable of detecting pulmonary and extrapulmonary TB.
The XPHACTOR “Investigating TB if initial Xpert is negative” dataset, which includes data underlying this substudy, has been uploaded to the LSHTM Data Compass repository: https://doi.org/10.17037/DATA.28421.
The reader will need to request the dataset from LSHTM (request access is provided within the data record) with a brief summary of how the dataset will be utilised. On request, a data sharing agreement will be made available which will first need to be signed, prior to provision of the dataset. This enables LSHTM to confirm that the reader is using the data for HIV or TB-related research, which is required because study participants consented to use of their data for HIV or TB-related research only.
The data is shared under a Data Sharing Agreement license (see above).
The study team wish to avoid unnecessary barriers to access and will seek to respond to data requests as quickly as possible.
We thank the study participants; the nursing and medical staff of Chris Hani Baragwanath and Mamelodi hospitals, Ramokonopi and Jabulani Dumane community health clinics, South Africa; the staff of National Health Laboratory Services, South Africa; and the staff of Aurum Institute for their essential contributions to this study.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: infectious diseases
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 25 Feb 22 |
read | ||
|
Version 1 26 Apr 18 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)