Keywords
Karachi, Heath and Demographic Surveillance System, HDSS, maternal and child health, longitudinal studies.
Karachi, Heath and Demographic Surveillance System, HDSS, maternal and child health, longitudinal studies.
Pakistan has a national database for registering vital events, such as births and deaths, but coverage is sub-optimal with many births and deaths going unrecorded. Undercounting of these events leads to inaccurate estimates of vital health indicators. This inaccuracy hinders setting up of priorities and allocation of scarce resources at a national level. The Health and Demographic Surveillance System (HDSS) was established in 2003 by the Department of Paediatrics and Child Health of the Aga Khan University, Karachi, Pakistan, in peri urban areas of Karachi, with the mandate to ameliorate some of these gaps. The HDSS provided a research platform for both observational and interventional studies that could influence decision-making and planning for health strategies at local, national and international levels, as well as research training opportunities for students.
At the outset, various epidemiological studies were conducted in the area on infectious diseases of children, vaccine coverage and the impact of multiple interventions. This article provides detailed information about set up of this surveillance system, data collection methods, studies that were completed, and on-going and future plans.
Karachi is the largest metropolitan city, a commercial hub and a principal port of Pakistan, with an estimated population of 14.9 million including peri-urban areas (http://www.pbscensus.gov.pk). Located by the Arabian Sea, HDSS was established in four peri-urban low income communities in Korangi and Bin Qasim towns. Of the four peri-urban communities, three are contiguously located along the sea coast of Karachi; Ibrahim Hydri, Ali Akbar Shah Goth and Rehri Goth (Figure 1). The main occupation of people living in these communities is fishing. The fourth community, Bhains colony, is located at the outskirts of Karachi and the source of their livelihood is cattle rearing. In recent years Bhains colony has shown rapid urbanization.
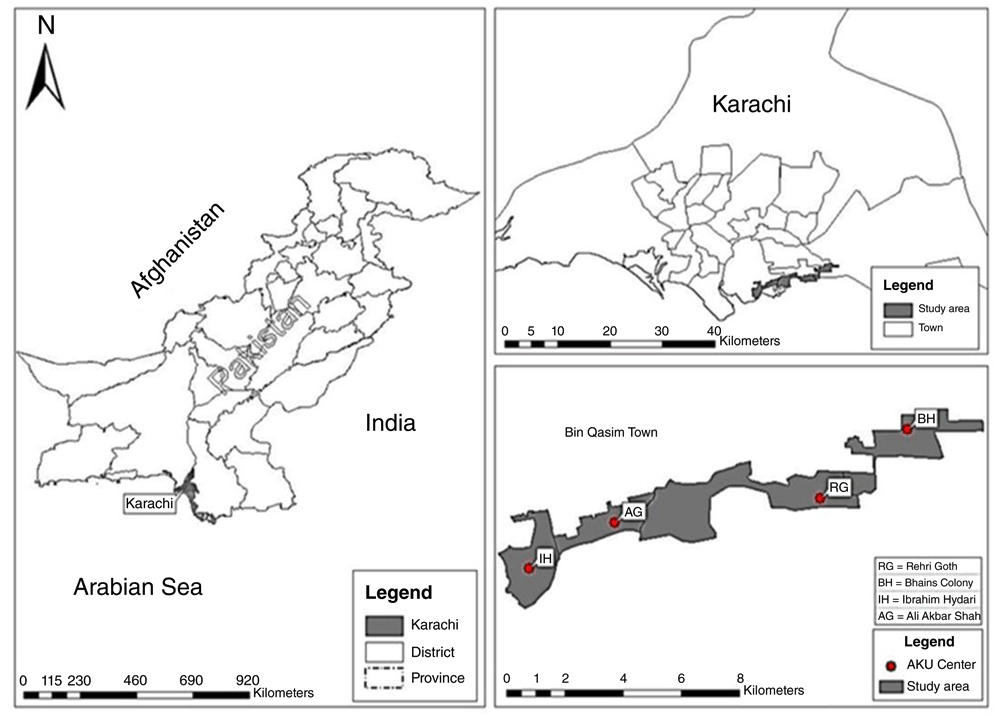
In 2010, the area was digitally mapped using global positioning system (GPS) techniques and boundaries were constructed. KHDSS lies on the latitude 24.8508° N and longitude 67.0181° and covers an area of 17.6 square kilometres.
The Surveillance sites have been divided into 195 blocks each containing about 200–250 structures. There are a total of 42,093 structures where 43,098 households are living. A ‘structure’ is defined as a building with a single entrance and a boundary. These structures can be houses, hospitals, dispensaries, schools, shops, parks etc. Each structure has a unique number assigned to it. A ‘household’ is a group of people living together under a roof (structure) and sharing the same cooking pot. A ‘resident’ is defined as a person who stayed for at least six months or intends to stay for more than six months in the community.
Until 2014, quarterly enumeration was done. After 2014, this was intensified to every two months, to be done by trained community health care workers (CHWs). CHWs are women with a secondary level of education and are mostly residents of the same communities where surveillance is taking place. At each two monthly re-enumeration, CHWs move through the area using GIS-derived maps and collect the information from households. If a household has married women of child bearing-age, any new pregnancy is documented and followed. All the information is documented on a printed form, which has a specific section for entering data about pregnancy status, any in/out-migration and newly born child to the household. If a pregnancy within a household has been identified in the previous rounds, the interviewer affirms if that woman is still pregnant or her pregnancy has ended in a live- or still-birth.
In HDSS, only married women of child bearing age (15 to 49 years) and children under the age of five years are being identified and followed. Verbal consent is taken for inclusion in HDSS and a unique identification number (ID) is assigned. For inclusion into subsequent studies/trials, written informed consent is taken as per the ERC requirement. For the purpose of HDSS, information is collected from these married women about their pregnancy status and birth outcomes. Information is also collected about in/out migration, adult female deaths and deaths of children under five years of age. Additionally, a census is conducted every five years.
When a married woman is found to be pregnant at least four visits are made during pregnancy to closely ascertain the outcome. Upon a live birth, the newborn is assessed for the World Health Organization (WHO)’s seven danger signs and is referred to the site’s primary health centre for management and/or further referral. All newborns are followed at day 0, 3 and 10 after the birth and subsequently every two months. In case of a stillbirth, child death (under 5 years of age) or adult female death, Verbal Autopsy (VA) is conducted by a senior research assistant (with a Masters level degree in Sociology or related field). VAs are later analysed by physicians to determine the cause of death.
Each site has its own primary health care (PHC) centre that has been established and operated by the Aga Khan University Department of Paediatrics and Child Health. These PHCs are accessible to populations within their catchment area and provide free care to children under five years of age.
We have an integrated computerized system for entering and storing data. Core system was designed and developed in-house using MS Access (Microsoft Corporation). It consists of data entry screens, data edit and update screens, customized reports generation and data cleaning modules. The data is maintained at a central server at the Paedatrics Research Office, located about 25 kilometres from the field sites. A backup is also created.
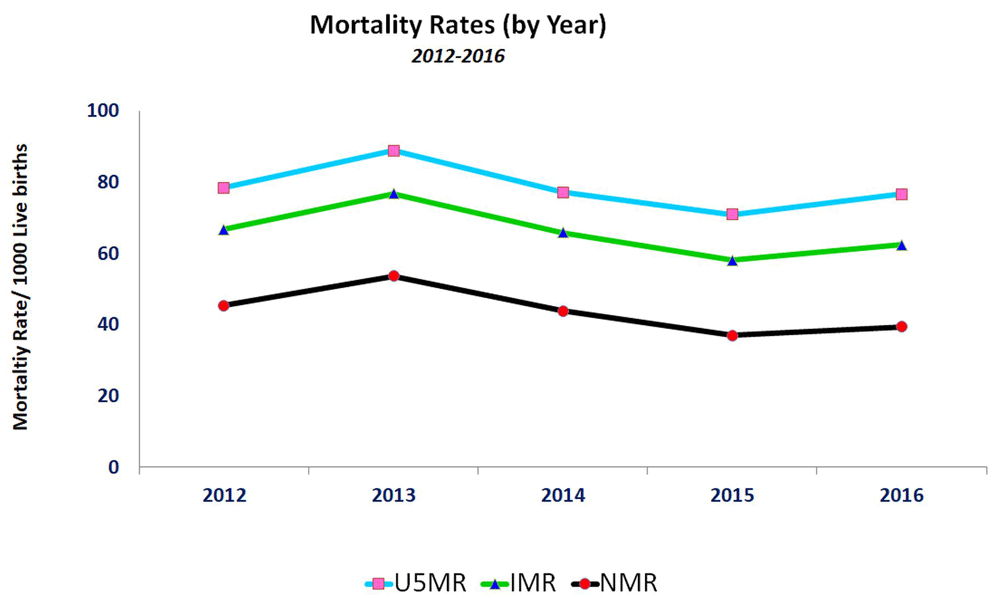
Since the completion of baseline census in 2010, we have been recording demographic information about vital events, migration patterns and various other socioeconomic factors. The demographic characteristics for the year 2016 are summarized in Table 1. The current estimated population is 249,128. Of these, 129,546 (52%) are males and 119,581 (48%) are females. Of the total population, 25% consist of females of reproductive age (between 15 to 49 years) and 16% consist of children under five years of age. The main demographic indicators have been represented in Table 2 for a period of five years. Figure 2 shows the trends of under 5 child mortality in the DSS from year 2012–2016. Under 5 mortality rates peaked in 2013 and 2016 due to measles epidemic (data not shown). Within the time period of five years, a reduction in neonatal mortality rates has been observed. (Table 2 and Figure 2).
HDSS allows for an efficient, cohesive and dynamic surveillance system. Some of the initial studies included identifying signs and symptoms in young infants requiring urgent referral and measuring the incidence of vaccine-preventable diseases such as rotavirus associated diarrhea, pneumonia, invasive pneumococcal disease, typhoid bacteremia and diseases, such as omphalitis and their contribution in causing neonatal mortality1–4.
Additional studies, added later, included, studying etiology of moderate to severe diarrhea (Global Enteric Multicenter Study or GEMS), comparison of effectiveness of different antibiotic regimens given as an outpatient therapy for management of sepsis in young infants (Simplified Antibiotic Therapy Trial or SATT) and a community-based etiology study of possible serious bacterial infections in young infants (0–59 days)(Aetiology of Neonatal infection in South Asia or ANISA)5–7.
Currently, ongoing studies include exploring coverage of routine childhood immunizations and their impact on disease transmission, e.g. impact of 10 valent pneumococcal vaccine on nasopharyngeal carriage, a randomized control trial (RCT) (NCT02372461) for comparison of Amoxicillin and placebo in non-severe pneumonia in children 2 to 59 months old (RETAPP), and usefulness of thermal images in diagnosing pneumonia in children under five years of age. In order to understand the intergenerational effects of disease across the continuum of adult female, maternal and child health, relevant large cohort studies were added, Alliance for Maternal and Newborn Health Improvement study (AMANHI)8–15.
HDSS has been proved as an important platform for carrying out various public health projects. Several studies done at HDSS have led the efforts to aid policy makers in making important decisions. One of these studies was the Young infant clinical signs study (YICSS), which led to the formulation of WHO seven sign algorithm for detection of possible serious bacterial infection (PSBI) in young infants. This algorithm was then incorporated into the Integrated Management of Childhood Illness (IMCI) and is in use to date1.
An RCT (NCT00189384) compared (1) procaine-penicillin and gentamicin, (2) ceftriaxone and (3) trimethoprim-sulfamethoxazole (TMP-SMX) regimens for the treatment of newborns, aged 0 to 59 days, with PSBI in an outpatient setting, when hospitalization is declined. TMP-SMX showed the highest failure rate and case fatalities. Procaine penicillin-gentamicin turned out to be the most cost effective route to treat these bacterial infections8.
As a follow up to this trial, a randomized control open-label equivalence trial (SATT) (NCT01027429) in young infants with clinically diagnosed severe infections (CSI), seen at PHC, was done. The trial aimed to evaluate if (1) IM gentamicin once daily (OD) and oral amoxicillin twice daily (BD) for 7 days; and (2) IM penicillin and gentamicin OD for two days followed by oral amoxicillin BD for five days are equivalent to seven days of (3) IM procaine penicillin and gentamicin (reference therapy). The primary outcome of this trial was treatment failure (death, deterioration or lack of improvement) within seven days of enrollment. Treatment failure rate were equivalent across three regimens. These findings were subsequently incorporated in the WHO guidelines for the management of young infants with CSI, and IM gentamicin OD and oral amoxicillin BD for 7 days was chosen as the treatment of choice6.
A study, Global Enteric Multicenter Study (GEMS), exploring etiology of moderate to severe diarrhea, using quantitative molecular diagnostic methods showed Shigella spp, Rotavirus, Adenovirus 40/41, ST-ETEC, Cryptosporidium spp, and Campylobacter spp are responsible for 77.8% of all diarrheal causes5.
In another study, health seeking behavior for sick young infants was studied. The acceptance rate of hospitalized care was found to be 24%. Reasons for high refusal rate included financial difficulties, elders denying permission and some based their decisions on religious and cultural beliefs. The acceptance of hospitalization was higher when the mother recognized the severity of the illness, presence of grunting, temperature <35.5°C and absence of language barrier at the local hospital. Gender was not a determining factor in decision making. This information forms learning points for interventions promoting health seeking behavior and formulation of alternative community based management plans for the betterment of child survival10.
An RCT aimed to compare immunization coverage by administering pictorial messages promoting vaccines to mothers versus administering general health promotion messages to the control group. An improvement of 39% in the completion of Pentavalent vaccines was seen in the intervention group, which shows that simple health awareness interventions can go a long way in raising the health status of low-income communities16.
A randomized double blinded placebo-controlled equivalence trial (MATT; NCT01533818) was conducted in primary care settings, which aimed to determine optimal management of isolated fast breathing in young infants. The primary objective of the study was to evaluate if out-patient therapy of seven days of oral amoxicillin (reference therapy) is equivalent to the placebo. The primary outcome was to see the treatment failure by evaluation of hypoxia, organ failure, anaphylaxis or hospitalization after treatment initiation. Amoxicillin treatment regimen was found to be more effective than placebo with risk difference of 3.1, p=0.04 (95% CI 0.3, 5.8)17.
A cohort study on calculating the neonatal mortality within 24 hours of birth was conducted in the rural areas of six countries, including HDSS in Pakistan. The neonatal mortality rates were higher than the published model-based estimates for these countries. Around one third of the deaths occurred during first six hours after birth and a little under half of all neonatal deaths within 24 hours. The study concluded that implementing high quality obstetric and newborn care is a priority for preventing newborn deaths early on18.
An RCT (NCT01695798) was conducted to see the immunogenicity of poliovirus vaccines in chronically malnourished infants. Infants were randomized to receive one dose of either bivalent oral poliovirus vaccine (bOPV) alone or in combination with inactivated poliovirus vaccine (IPV). The results showed that those who were given bOPV+IPV together showed to close the immunity gap more than those who were given bOPV alone19.
Future analysis plans include analysis of data from multi-center Aetiology of Neonatal infection in South Asia (ANISA) study7. We are currently doing analysis to determine the burden, timing and causes of stillbirths, neonatal and maternal deaths as part of the multicenter Alliance for Maternal and Newborn Health Improvement (AMANHI) study12. We are also conducting analysis to determine the burden of major maternal morbidities as part of the AMANHI study13. An additional analysis is on simplified methods to determine gestational age at birth by using a combination of physical and neurodevelopmental parameters15. Also as a part of AMANHI, we have established a bio-repository of maternal, newborn and paternal samples, collected at various time points during and after pregnancy14. To the best of our knowledge, this is the only population based bio-bank in Pakistan and one of the few in the region. Recently, we have secured funding from the Bill and Melinda Gates Foundation to follow the AMANHI bio-bank cohort for up to three years for neurodevelopmental milestones.
In our DSS, all inhabited and uninhabited structures in the area have been mapped and all women of reproductive age and children under the age of five years have been assigned a unique ID. The list is continually updated every two months. We have GIS coordinates of all structures, which allows us to look at spatial distribution of various maternal and newborn health indicators. Active surveillance of maternal and child health allows for the cohort to be a part of many multicenter studies, conducted with multiple international collaborators. Our long term presence in the area has helped us establish good rapport with the population resulting in very low refusal rates.
Currently, the HDSS covers only children under the age of five years and women of reproductive age. Older children, male adults and unmarried women are not followed. In the future, given adequate amount of funding we would like to expand our surveillance to cover these populations as well. Currently, we are using paper based forms for data collection. Increasing availability of modern technologies, like smartphones and tablets, provide an opportunity to move data collection to digital platforms.
All the studies conducted at our surveillance sites aim for improvement of public health policies. The information we derive, aid us in making informed decisions at local, national and international levels. These sites also play vital roles in training research personnel.
Data gathered from HDSS can be shared with other investigators with similar research interests upon receiving reasonable requests in the form of a proposal. All personal identifiers and addresses will be removed. Data sharing with other demographic surveillances provides an opportunity to learn and understand geological differences. Data sharing requests can be sent to Aga Khan University via Muhammad Imran Nisar (imran.nisar@aku.edu).
Bill & Melinda Gates Foundation [OPPGH5307, OPP1033572].
Dr Imran Nisar and Dr Fyezah Jehan were supported by grant number 1 D43 TW007585-01 from the National Institute of Health’s Fogarty International Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Benazir Balouch Muhammad Sajid, Najeeb Rehman, Saima Jamal, Nasira Hina, Rehana Bashir, Saima Jameel, Taj Muhammad, Fakhra Parveen, Lubna Aziz, Gul Froze, Fatima Ali, Aftab Zaidi, Asghar Ali, Salim Charania, Shukurullah Baig, Muhammad Siddique, Asad Khowaja, Faraz Hussain, Mir Hakim, Azhar Abbas, Ahmed Ali.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Demography
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: population health; health and demographic surveillance systems
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology, demographic surveillance, population health measurement, equity, social determinants of health
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 08 Dec 22 |
read | |||
|
Version 1 04 Jan 18 |
read | read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)