Keywords
Levonorgestrel Intrauterine System (LNG IUS), contraceptive method discontinuation, contraceptive method mix, Nigeria Family Planning.
Levonorgestrel Intrauterine System (LNG IUS), contraceptive method discontinuation, contraceptive method mix, Nigeria Family Planning.
Discontinuation of contraception among women in Nigeria is one of the main challenges with contraceptive use, together with generally low use of contraception. The recent national demographic health survey report (NDHS 2018) indicates that about 41% of women who had an episode of use of contraception discontinued the contraception within 12 months of use1. Contraceptive discontinuation and dissatisfaction with a method occur due to several reasons which may be method related. While the desire to become pregnant is one of the major reasons why a woman will discontinue contraception, method-related reasons such as side effects, method failure, and the need for a more effective method are also prominent reasons for method discontinuation among women who still wish to prevent pregnancy. Discontinuation of contraceptives often leads to unwanted pregnancies and reduces the effort of family planning (FP) programs2. Studies show that a sizable proportion of women become exposed to the risk of unintended births after discontinuation of contraceptives3,4. In Nigeria, it has been reported that an estimated 15.6% of unintended pregnancies are among women who discontinued contraception in the last seven months4. Even higher proportions of unintended births have also been reported in other countries as a result of the discontinuation of contraceptives in the last three months3. Serious reproductive consequences of discontinuation because of reported method failure and method-related reasons can occur. Accidental pregnancies that end in miscarriage, stillbirth, or abortion have been reported3
In many developing countries including Nigeria, many health outlets providing services for modern contraception are highly constrained on the method mix available for potential users5. It is widely known that limited method choice can hinder the uptake and use of contraception, and has been linked with dissatisfaction and discontinuation of contraception6,7. The modern contraceptive method mix in Nigeria comprises mainly injectable contraceptives (33.8%), pills (23%), male condoms (18.1%), Implant (10.9%), and intra-uterine devices (IUDs) (5.2%). Other methods such as the lactational amenorrhea method (LAM) make up about 4.8%, while female and male sterilization make up 1.6% and 0.2% of the modern contraceptive method mix, respectively8. Global data is available on the dynamics of contraceptive use as it relates to the discontinuation of methods. According to data from 19 different Demographic Health Survey (DHS) participating countries, an average of 38% of women reported discontinuing their use of reversible methods by the 12th month and 64% by the 36th month3. The lowest 12-month discontinuation rate was noted for the intrauterine device (IUD; 13%) and the highest was for condoms (50%), while the pill and injectable contraceptives were discontinued by about 40% of users within the first 12 months of use. Contraceptive continuation indicates the acceptability of a contraceptive method, and contraceptive continuation rate is the cumulative probability that acceptors of a contraceptive method will still be using any contraceptive method offered by a program after a specified period9. Given this understanding, a high continuation rate of a method indicates users' high level of compliance with the method.
From the pattern of contraceptive method-use according to the Nigeria 2018 National Demographic Health Survey, the national prevalence of 5% of IUD users, use mainly the copper IUD, which is the most common intrauterine contraceptive available in FP clinics in Nigeria. Meanwhile, other types of intrauterine devices such as the hormonal IUD which is a progestin-based intra-uterine delivery system and is a long-acting and reversible contraceptive (LARC), also exist. However, the hormonal IUD does not contribute to the proportion of intrauterine contraceptives used by women10. In one study in Zaria Nigeria, of all the 1104 women who opted for an IUD as a method of contraception only 68 (6.1%) women chose the hormonal IUD11. This is in contrast to other countries; for example in the US, where the proportion of IUD users has been reported as 12%, with 74% of the users of the intrauterine contraceptives chosing the hormonal IUD12. In Nigeria, the use of the hormonal IUD is limited and is widely unavailable to users of contraceptives from both public and private clinics. The limited use of the method is mainly due to its high cost; a unit of the LNG IUS brand Mirena®, which is manufactured by Bayer Healthcare Pharmaceuticals Inc., costs between 25,000 to 90,000 Nigerian Naira, which is currently equivalent to about $69 – $250, compared with the copper IUD, which costs about $5 or less at private clinics and is free of charge in government clinics. In addition, the hormonal IUD method has been relatively recently introduced in the country despite its decades of existence since its development in the 1970s4,13. There is also the issue of the availability of providers who are trained to provide the method, thus limiting its use.
The hormonal IUD is a T-shaped device comprising a cylinder containing 52 mg of levonorgestrel, with an average release of 14µg of levonorgestrel every 24 hours of the life of the IUS14. The Mirena® LNG IUS that is produced by Bayer Healthcare Pharmaceuticals Inc is indicated to have an effective duration of up to 5 years. However, clinical trials suggest that the LNG IUS can provide contraceptive protection for up to 7 years15,16. From a recent study trial, a brand of the IUS Lilelleta developed by Medicine360 (registered under the trade name as AvibelaTM in the FP2020 countries including Nigeria), which was previously labeled for use for 3 years has also been indicated to have an effective duration over five years17. Eliora another brand of the hormonal IUD developed by Pregna is similar to the Mirena having an effective duration of 5 years18. The IUS functions locally in the uterus by thickening the cervical mucus and suppressing endometrial proliferation, thus inhibiting conception. The IUS is known to have both contraceptive and non-contraceptive health benefits in the treatment of menorrhagia, endometriosis, and endometrial hyperplasia14. The method is very convenient as it requires nothing else to be done regularly to maintain contraception until after five years when the device can be replaced. Most side effects of the IUS are transient and include changes in menstrual bleeding pattern, headache, abdominal cramps, breast tenderness, and weight gain13.
To expand the range of contraceptive options available in the country and to improve the acceptability of contraception, the introduction of new and effective methods such as the hormonal IUS as a strategy to increase contraceptive uptake has been the focus of some FP interventions and organizations that are piloting roll out of new and affordable IUS to potential users in Nigeria. For instance, since 2017 the Society for Family Health (SFH) has been supporting service provision for the LNG IUS as part of a broad range of options available in 40 private health clinics across 17 states in Nigeria, using donations of the registered product in Nigeria from the International Contraceptive Access (ICA) Foundation. To better understand the potential of the hormonal IUS method to contribute to the contraceptive method mix in Nigeria, this study was therefore undertaken to assess and document the continuation of use of the LNG IUS and satisfaction with the method, as well as user characteristics and experiences that may influence continuation and satisfaction among the user population. This will add to the existing body of knowledge on the method and document the experiences of users of the method in Nigeria. If users show high satisfaction and continuation with the IUS, these findings will go a long way to encourage adoption and utilization of the method by users and provide the motivation for its recommendation by FP providers and clinicians, both as a contraceptive and for its non-contraceptive benefits such as treatment of menorrhagia and other gynecological disorders.
The study protocol was reviewed by the National Health Research Ethics committee in 2017 and ethical approval was obtained (NHREC/01/01/2007-05/02/2017). Given that data collection in this study was done via phone interviews, oral informed consent was deemed adequate and was obtained from all participants. The consent to partake in the study was recorded by the researchers along with responses of respondents and a written consent form was signed by the interviewers for documentation.
This study was a prospective longitudinal survey of 209 women of reproductive age 18 – 49 years who received the LNG IUS as a method of choice after undergoing counseling using the Balanced Counselling Strategy (BCS) for FP. The study was conducted in 40 select private health clinics that are members of the SFH Healthy Family Network (HFN), a social franchise of private health facilities. The 40 selected facilities had high numbers of clientele with IUDs and are implementing service delivery for LNG IUS under the Supporting International Family Planning Organisation (SIFPO) Project, which provided the IUS method in the context of informed choice using the ICA Foundation donated LNG IUS product. The facilities are in 17 states in Nigeria, namely Abia, Akwa Ibom, Cross River, Enugu, Rivers, Kano, Katsina, Imo, Gombe, Taraba, Lagos, Ogun, Oyo, Benue, Niger, Edo, and the Federal Capital Territory (FCT) where the franchise operates.
Trained health providers in the facilities recruited consenting women who already accepted the IUS as their method of choice to participate in a telephone interview. Only women aged 18 to 49 years were recruited. Recruited participants were contacted by trained call agents/interviewers at predetermined periods: at two weeks post-insertion of the IUS in the facility, at three months, and after 12 months. Women who consented and agreed for their phone number to be passed on to the research team were contacted for interviews and follow-up. Only their first names and phone numbers were passed to the research team via SMS. The SMS was immediately deleted from the provider’s phones after receipt from the research team. Women less than 18 years of age were excluded from this study. Confidentiality of the respondent’s information was ensured by assigning unique identification codes to each recruited participant, and all respondents’ data were electronically stored, and the files were password protected. The sample size calculation for the study was based on the following formula:
Where N is the sample size, P1 (proportion of the sample reporting satisfaction at time 1) = 75%, P2 (proportion of the sample reporting satisfaction at time 2) = 60%, Z1- α is the standard normal deviate corresponding to a two-sided level of significance (α) of 5%, Z1-β (statistical power) = 0.8. The initial calculated sample size was 135 considering a design effect of 1.2. The final sample of 208 was recruited with the consideration of about 45% loss-to-follow-up rate between baseline and the 12-month follow-up.
Women were considered as loss-to-follow up if they could not be reached after at least five repeated attempts within a couple of days. The loss-to-follow-up was mainly due to non-reachable phone contacts after five repeated attempts and due to women refusing interviews during the follow-up. No form of incentive was given to participants in the study. Baseline data collection started in May 2017 and lasted for about 8 months until the required sample was achieved. Subsequent follow-up interviews were done on a date scheduled with the participant at three months and 12 months. Data collection, therefore, ended in 2018 after the last scheduled 12-month interview. Data were collected electronically using a questionnaire implemented on the ODK android software. The ODK is a computer-assisted personal interview (CAPI) application available for Android devices for electronic data collection.
Information was collected on the respondent’s background characteristics and their FP use experience, which included pregnancy intention, the occasion for the insertion of the IUS such as miscarriage or abortion, side effects (experienced during the use of IUS at three months and 12 months) and complaints of discomfort from the IUS string by her partner. The women were also asked about how satisfied they were with using the IUS, and if they would recommend the IUS to other women or a friend. A Likert scale was used to score the responses of the women to the satisfaction question. The Likert scale ranged from 1–5, with an increasing score indicating increasing satisfaction with the method. User satisfaction response was re-coded such that a respondent’s score of 1–3 indicated no satisfaction, while scores of 4–5 indicated satisfaction with the method. Continuation rate was measured as the proportion of women who received the IUS and reported they are still using the method at the time of the three-month and 12-month follow-up surveys.
Data was presented in this study using descriptive statistics with frequencies, percentages, and 95% confidence intervals as appropriate. Association between independent variables of background characteristics and FP/ IUS experiences with the dependent variables (continuation of the IUS and satisfaction) were tested using the Chi-square test. Statistical significance for association was set at p<0.05. Given the attrition recorded in the survey, possible attrition bias was examined by carrying out a Chi-square test to compare the background characteristics between those who were reached in the second and third rounds of the study with those who were not reached. Statistically significant differences in the background characteristics between those reached and those not reached would indicate some existence of attrition bias in the result estimates. Data analysis was done using IBM SPSS Statistics - version 25.
A total of 208 users were interviewed at baseline. At the three-month follow-up, 98 women were successfully reached and interviewed, while at the 12-month follow-up, 73 women were successfully reached and interviewed. The average age of users of the IUS in the study at baseline was 33.7±6, and the majority age group was 35 years and older (44.2%), while approximately 30% and 20% of the users were aged between 30–34 years and 25–29 years old, respectively. More than half of users had between 1–3 children (58.2%), another good proportion, 38.5%, had 4–7 children, and very few users had no children (1.4%) (Table 1). The Chi-square test results in Table 1 showed statistical significance for only age at 3-month for the sample due to the attrition. For all other background characteristics, no statistical significance was observed in the background characteristics of the participants reached and those not reached in the follow-up surveys. Nearly a third of the women in the study reported they were no longer seeking pregnancy (31.7%), while another 23.6% said they would seek a pregnancy in the next 3–5 years, and 10% said in the next 1–2 years.
Around 4% reported receiving the IUS two weeks before carrying out an abortion or after a miscarriage. Up to 96.2% of users of the method reported they were satisfied with getting the IUS inserted at the clinic at the uptake of the method. Table 2 presents more of the IUS use experiences and characteristics of the participants.
The most common side effects were less or no bleeding, irregular bleeding, and breast tenderness/pain. Reports of less bleeding increased from 8.2% at three months to 24.7% at 12 months, while irregular bleeding which was high at 3 months (32.7%) reduced to 4.1% at 12 months. The prevalence of other side effects during the use of the IUS at three months and 12 months are presented in Table 3.
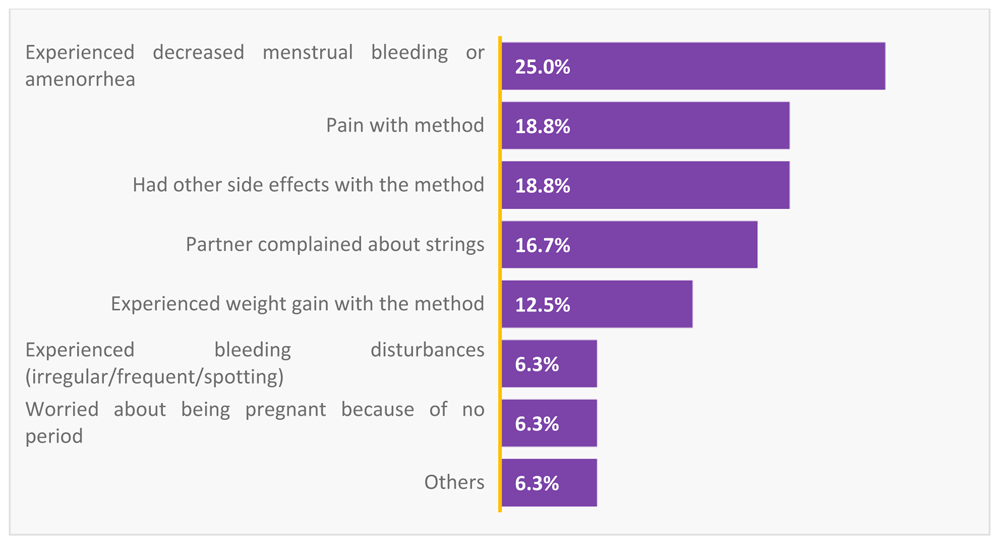
Some of the reasons for discontinuation of the IUS at 12 months were mainly due to experience of decreased menstrual bleeding or amenorrhea (25%), pain (18%), and weight gain (12%). Other reasons were as a result of worry of being pregnant because of no bleeding (6.3%) and partner complaining about feeling the strings of the IUS (16.7) (see Figure 1).
Concerning recommending the benefits of the IUS, a high rate for a recommendation of the benefits of the IUS were seen for attributes such as its fewer sides effects (23.4% at 3 months and 14.4% at 12 months) and its convenience of use (21.9% at 3 months and 15.1% at 12 months). Other reported high reasons for recommending the IUS were its high effectiveness, long duration of effectiveness, reduced bleeding, and affordability of the product at the facility (Table 4).
Of the 98 women who were interviewed at three months post-insertion of their IUS, 96.9% (95% CI: 91.3, 99.3) of them were still using the method, while at 12 months post-insertion, 91.8% (95% CI: 82.9, 96.9) of the 73 women reached reported still using the IUS method. The proportion of users who were satisfied with the method at baseline at two weeks post-uptake was 95.2% (95% CI: 91.3, 97.6), while satisfaction with the method at three months and 12 months post-uptake was 76.5% (95% CI: 66.8, 84.5) and 86.3% (95% CI: 77.5, 94.1), respectively.
Our analysis showed that continuation of IUS at 12 months was significantly lower (83.8%) among women who visited a provider because of problems with the method than women who did not visit a provider (100%). Analyses were also conducted to see if continuation was correlated with age, fertility intention, counseling, etc. but none of these were found to be statistically significant. In addition to the higher rate of uptake among older women, the continuation rate at 12 months was higher among older women of ages 30 years and older, ranging from about 92% to about 97%, compared with those aged 25 – 29 years old, which was about 80% (Table 5). According to marital status, high rates for the continuation of the IUS were seen at three and 12 months post-insertion among women living with their partner, which was 96.8% at 3 months and 91.7% at 12 months. As there were not enough single women in the study, the continuation rate among this group was not presented. Continuation of the use of IUS based on fertility intention was varied among users but higher among users who intended to get pregnant after three years than those intending to become pregnant in under 3 years; this was, also not significant (p>0.05). Counseling on side effects was also not statistically associated with continuation of IUS, although more women who received counseling on side effects (95.5%) than those who did not receive counseling (90%) continued the use of the IUS at 12 months.
| User Characteristics | 3 months post insertion | 12 months post insertion | ||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Total | X2(p) | Yes | No | Total | X2(p) | |
| n (%) | n (%) | n (%) | n (%) | |||||
| Age category (baseline) | ||||||||
| 18–24 years old | 1 (100.0) | 0 (0.0) | 1 | 2.722 (0.436) | 1 (100.0) | 0 (0.0) | 1 | 3.822 (0.281) |
| 25 – 29 years old | 20 (95.2) | 1 (4.8) | 21 | 12 (80.0) | 3 (20.0) | 15 | ||
| 30 – 34 years old | 30 (93.8) | 2 (6.2) | 32 | 25 (92.6) | 2 (7.4) | 27 | ||
| 35 and older | 44 (100.0) | 0 (0.0) | 44 | 29 (96.7) | 1 (3.3) | 30 | ||
| Level of schooling (baseline) | ||||||||
| Never attended school | 1(100.0) | 0 (0.0) | 1 | 1.824 (0.402) | - | - | ||
| Attended but did not complete school | - | - | ||||||
| Primary | 9(90.0) | 1 (10.0) | 10 | 7 (100) | 0 (0.0) | 7 | 0.693 (0.405) | |
| Secondary or higher | 85 (97.7) | 2 (2.3) | 87 | 60 (90.9) | 6 (9.1) | 66 | ||
| Marital status (baseline) | ||||||||
| Single | - | - | - | - | - | |||
| Married/living together | 93 (96.8) | 3 (3.1) | 96 | 0.064 (0.968) | 66 (91.7) | 6 (8.3) | 72 | 0.091 (0.763) |
| Widowed | 1 (100) | 0 (0.0) | 1 | 1 (100) | 0 (0.0) | 1 | ||
| Divorced/separated | 1 (100) | 0 (0.0) | 1 | |||||
| Religion (baseline) | ||||||||
| Islam | 13 (92.9) | 1 (7.1) | 14 | 1.211 (0.546) | 8 (88.9) | 1 (11.1) | 9 | 0.143 (0.931) |
| Christian (non-Catholic) | 69 (97.2) | 2 (2.8) | 71 | 49 (92.5) | 4 (7.5) | 53 | ||
| Christian (Catholic) | 13 (100) | 0 (0.0) | 13 | 10 (100) | 0 (0.0) | 10 | ||
| Parity (baseline) | ||||||||
| 0 | 0 | 0 | 0 | - | - | - | ||
| 1 | 7 (100) | 0 (0.0) | 7 | 0.326 (0.955) | 4 (66.7) | 2 (33.3) | 6 | 7.224 (0.065) |
| 2 | 28 (96.6) | 0 (3.4) | 29 | 19 (95.0) | 1 (5.0) | 20 | ||
| 3 | 24 (96.0) | 1 (4.0) | 25 | 19 (100) | 0 (0.0) | 19 | ||
| 4 and more | 36 (97.3) | 1 (2.7) | 37 | 25 (89.3) | 3 (10.7) | 28 | ||
| Age of youngest child (baseline) | ||||||||
| 0 year | 34 (97.1) | 1 (2.9) | 35 | 0.319 (0.853) | 26 (92.9) | 2 (7.1) | 28 | 0.659 (0.719) |
| 1–5 years | 53 (96.4) | 2 (3.6) | 55 | 36 (90.0) | 4 (10.0) | 41 | ||
| 6 years and above | 8 (100) | 0 (0.0) | 8 | 5 (100.0) | 0 (0.0) | 5 | ||
| Pregnancy intention (baseline) | ||||||||
| In less than a year | - | - | - | - | - | - | ||
| In 1–2 years | 11 (100) | 0 (0.0) | 11 | 2.930 (0.570) | 8 (88.9) | 1 (11.1) | 9 | 1.973 (0.741) |
| In 3–5 years | 21 (95.5) | 1 (4.5) | 22 | 16 (94.1) | 1 (5.9) | 17 | ||
| In more than 5 years | 5 (100) | 0 (0.0) | 5 | 3 (100.0) | 0 (0.0) | 3 | ||
| Do not know | 30 (100) | 0 (0.0) | 30 | 22 (95.7) | 1 (4.3) | 23 | ||
| (Never) | 28 (93.3) | 2 (6.7) | 30 | 18 (85.7) | 3 (14.3) | 21 | ||
| Receiving IUS within 2 weeks of having an abortion or miscarriage | ||||||||
| Yes | 2 (100) | 0 (0.0) | 2 | 0.064 (0.800) | 1 (100.0) | 0 (0.0) | 1 | 0.091 (0.763) |
| No | 93 (96.9) | 3 (3.1) | 96 | 66 (91.7) | 6 (8.3) | 72 | ||
| Impact of not bleeding to wellbeing# | ||||||||
| Positive | - | - | - | - | 5 (100.0) | 0 (0.0) | 5 | 1.714 (0.424) |
| Negative | - | - | - | - | 5 (71.4) | 2 (28.6) | 7 | |
| Neutral | - | - | - | - | 5 (83.3) | 1 (16.7) | 6 | |
| Partner feels IUS strings | ||||||||
| Yes | - | - | - | - | 17 (94.5) | 1 (5.6) | 18 | 0.572 (0.751) |
| No | - | - | - | - | 47 (90.4) | 5 (9.6) | 52 | |
| Do not know | - | - | - | - | 3 (100) | 0 (0.0) | 3 | |
| Counselling at uptake about what to do if side-effect is experienced | ||||||||
| Yes | 33 (97.1) | 1 (2.9) | 34 | 0.036 (0.982) | 21 (95.5) | 1 (4.5) | 22 | 0.693 (0.707) |
| No | 61 (96.8) | 2 (3.2) | 63 | 45 (90.0) | 5 (10.0) | 50 | ||
| Do not know | 1 (100) | 0 (0.0) | 1 | 1 (100) | 0 (0.0) | 1 | ||
| Visits to provider because of problem with method | ||||||||
| Yes | 19 (100) | 0 (0) | 19 | 0.315 (0.574) | 31 (83.8) | 6 (16.2) | 37 | 6.361 (0.012) |
| No | 60 (98.4) | 1 (1.6) | 61 | 36 (100.0) | 0 (0.0) | 36 | ||
| Total | 95 (96.9) | 3 (3.1) | 67 (91.8) | 6 (8.2) | ||||
Concerning the relationship between satisfaction with method and women’s characteristics (Table 6), our result showed that a significantly higher proportion of women, at 12 months, who did not visit a provider because of problems with the method (97.2%) were satisfied with the method compared with those who visited a provider (approximately 76%). This association also showed statistical significance a 3-months post-insertion. Satisfaction was significantly associated with religious affiliation at 3 months. We found significantly higher satisfaction (p<0.05) with the use of the IUS among users who did not report vaginal bacterial infection (87.5% satisfied) during the assessment at 12 months. The only case in the sample who reported vaginal infection did not report satisfaction with the method. Not experiencing breast tenderness or pain (90.8%) and not experiencing acne (88.4%) were also associated with satisfaction with the IUS, compared with experiencing breast tenderness and acne (50% each) with IUS use during the assessment at 12 months post-uptake (Table 7).
| User Characteristics | 2 weeks post-insertion (Baseline) | 3 months post-insertion* | 12 months post-insertion* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not satisfied | Satisfied | Total | X2(p) | Not satisfied | Satisfied | Total | X2(p) | Not satisfied | Satisfied | Total | X2(p) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||||
| Age category (baseline) | ||||||||||||
| 18-24 years old | 0(0) | 12(100) | 12 | 1.592 (0.661) | 0 (0.0) | 1(100) | 1 | 0.634 (0.889) | 0(0.0) | 1(100.0) | 1 | 1.038 (0.792) |
| 25 - 29 years old | 1(2.4) | 41(97.6) | 42 | 4 (19.0) | 17 (81.0) | 21 | 3 (18.8) | 12 (80.0) | 15 | |||
| 30 - 34 years old | 4(6.5) | 58(93.5) | 62 | 8 (25.0) | 24 (75.0) | 32 | 4 (15.4) | 23 (85.2) | 27 | |||
| 35 and older | 5(5.4) | 87(94.6) | 92 | 11 (25.0) | 33 (75.0) | 44 | 3 (10) | 27 (90.0) | 30 | |||
| Level of schooling (baseline) | ||||||||||||
| Never attended school | 0(0.0) | 2(100) | 2 | 3.842 (0.279) | 0 (0.0) | 1(100) | 1 | 3.800 (0.150) | - | - | - | |
| Attended but did not complete school | 1(25.0) | 3(75.0) | 4 | - | - | - | - | - | - | |||
| Primary | 1(6.2) | 15(93.8) | 16 | 0 (0.0) | 10 (100) | 10 | 1 (14.3) | 6 (85.7) | 7 | 0.002 (0.962) | ||
| Secondary or higher | 8(4.3) | 178 (95.7) | 186 | 23 (26.4) | 64 (73.6) | 87 | 9 (13.8) | 57 (86.4) | 66 | |||
| Marital status (baseline) | ||||||||||||
| Single | 0 (0.0) | 3(100) | 3 | 5.717 (0.126) | - | - | - | 0.626 (0.731) | - | - | - | |
| Married/living together | 9 (4.5) | 189 (95.5) | 198 | 23 (24.0) | 73 (76.0) | 96 | 10 (13.9) | 62 (86.1) | 72 | 0.161 (0.688) | ||
| Widowed | 1 (33.3) | 2 (66.7) | 3 | 0 (0.0) | 1 (100.0) | 1 | 0 (0.0) | 1(10.00) | 1 | |||
| Divorced/separated | 0 (0.0) | 4 (100) | 4 | 0 (0.0) | 1 (100.0) | 1 | - | - | - | |||
| Religion (baseline) | ||||||||||||
| Islam | 1 (2.7) | 36 (97.3) | 37 | 1.546 (0.462) | 2 (14.3) | 12 (85.7) | 15 | 6.119 (0.047) | 2 (20.0) | 7 (77.8) | 9 | 0.761 (0.683) |
| Christian (non-Catholic) | 7 (4.6) | 144 (95.4) | 151 | 21 (29.6) | 50 (70.4) | 71 | 7 (13.5) | 46 (86.5) | 53 | |||
| Christian (Catholic) | 2 (10) | 18 (90) | 20 | 0 (0.0) | 13 (100.0) | 13 | 1 (10) | 10 (90) | 11 | |||
| Parity (baseline) | ||||||||||||
| 0 | 1 (33.3) | 2(66.7) | 3 | 8.488 (0.075) | - | - | - | 3.822 (0.281) | - | - | - | 3.983 (0.263) |
| 1 | 2 (12.5) | 14 (87.5) | 16 | 1 (16.7) | 6 (85.7) | 7 | 2 (40.0) | 4 (66.7) | 6 | |||
| 2 | 3 (5.3) | 54 (94.7) | 57 | 7 (24.1) | 22 (75.9) | 29 | 4 (19.0) | 16 (80) | 20 | |||
| 3 | 1 (2.1) | 47 (97.9) | 48 | 3 (12.0) | 22 (88.0) | 25 | 1 (5.3) | 18 (94.7) | 19 | |||
| 4 and more | 3 (3.6) | 81 (96.4) | 84 | 12 (32.4) | 25 (67.6) | 37 | 3 (10.7) | 25 (89.3) | 28 | |||
| Age of youngest child (baseline) | ||||||||||||
| 0 year | 3 (4) | 72 (96.0) | 75 | 0.089 (0.957) | 6 (17.6) | 29 (82.9) | 35 | 2.284 (0.319) | 3 (11.1) | 24 (88.9) | 27 | 0.372 (0.830) |
| 1–5 years | 5 (4.8) | 99 (95.2) | 104 | 16 (29.1) | 39 (70.9) | 55 | 6 (15.0) | 35 (85.0) | 40 | |||
| 6 years and above | 1(3.8) | 25 (96.2) | 26 | 1 (12.5) | 7 (87.5) | 8 | 1(20) | 4 (80.0) | 5 | |||
| Pregnancy intention (baseline) | ||||||||||||
| In less than a year | 0(0.0) | 1 (100.0) | 1 | 10.732 (0.057) | - | - | - | - | - | - | ||
| In 1–2 years | 4 (19.0) | 17 (81.0) | 21 | 1 (10.0) | 10 (90.9) | 11 | 6.659 (0.155) | 2 (25.0) | 7 (77.8) | 9 | 2.937 (0.568) | |
| In 3–5 years | 2 (4.1) | 47 (95.9) | 49 | 6 (27.3) | 16 (72.7) | 22 | 3 (17.6) | 14 (82.4) | 17 | |||
| In more than 5 years | 0 (0.0) | 12 (100) | 12 | 0 (0.0) | 5 (100) | 5 | 0 (0.0) | 3 (100) | 3 | |||
| Never | 2 (3.0) | 64 (97.0) | 66 | 5 (16.7) | 25 (83.3) | 30 | 4 (17.4) | 19 (82.6) | 23 | |||
| Do not know | 2 (3.4) | 57(96.6) | 59 | 11 (36.7) | 19 (63.3) | 30 | 1(4.8) | 20 (95.2) | 21 | |||
| Receiving LNG IUS within 2 weeks of having an abortion or miscarriage (Baseline) | ||||||||||||
| Yes | 2 (25.0) | 6 (75.0) | 8 | 7.412 (0.006) | 1 (50.0) | 1(50.0) | 2 | 0.800 (0.371) | 0 (0.0) | 1(100.0) | 1 | 0.161 (0.688) |
| No | 8 (4.0) | 192 (96.0) | 200 | 22 (22.9) | 74 (77.1) | 96 | 10 (14.1) | 62 (86.1) | 72 | |||
| Impact of not bleeding to wellbeing# | ||||||||||||
| Positive | - | - | - | - | - | - | - | - | 0 (0.0) | 5 (100.0) | 5 | 1.714 (0.424) |
| Negative | - | - | - | - | - | - | - | - | 2 (28.6) | 5 (71.4) | 7 | |
| Neutral | - | - | - | - | - | - | - | - | 1 (16.7) | 5 (83.3) | 6 | |
| Partner feels IUS strings | ||||||||||||
| Yes | - | - | - | - | - | - | - | - | 3 (16.7) | 15 (83.3) | 18 | 0.613 (0.736) |
| No | - | - | - | - | - | - | - | - | 7(12.3) | 45 (86.5) | 52 | |
| Do not know | - | - | - | - | - | - | - | - | 0 (0) | 3 (100.0) | 3 | |
| Counselling at uptake about what to do if side-effect is experienced | ||||||||||||
| Yes | 3 (4.4) | 65 (95.6) | 68 | 0.318 (0.853) | 5 (14.7) | 29 (85.3) | 34 | 2.673 (0.263) | 1 (4.5) | 21 (95.5) | 22 | 2.500 (0.286) |
| No | 7 (5.1) | 128 (94.8) | 135 | 18 (28.6) | 45 (71.4) | 63 | 9 (18.4) | 41 (81.6) | 50 | |||
| Do not know | 0 (0.0) | 5 (100) | 5 | 0 (0.0) | 1 (100) | 1 | 0 (0.0) | 1 (100.0) | 1 | |||
| Visits to provider because of problem with method | ||||||||||||
| Yes | - | - | - | - | 3 (17.6) | 14 (82.4) | 17 | 7.002 (0.008) | 9 (24.3) | 28 (75.7) | 37 | 7.165 (0.007) |
| No | - | - | - | - | 1 (1.6) | 60 (98.4) | 61 | 1 (2.8) | 35 (97.2) | 36 | ||
| Total (N) | 10 (4.8) | 198 (95.2) | 208 | 23 (23.5) | 75 (76.5) | 98 | 10 (13.7) | 63 (86.3) | 73 | |||
| Side effects | Satisfaction with method at 12 months* | |||||
|---|---|---|---|---|---|---|
| % Not satisfied | % satisfied | Total | X2 | p-value | ||
| Less bleeding | No | 12.7 | 87.3 | 55 | 0.178 | 0.673 |
| Yes | 16.7 | 83.3 | 18 | |||
| No bleeding | No | 10.4 | 89.6 | 48 | 1.277 | 0.258 |
| Yes | 20.0 | 80.0 | 25 | |||
| Irregular bleeding | No | 14.3 | 85.7 | 70 | 0.497 | 0.481 |
| Yes | 0.0 | 100.0 | 3 | |||
| Vaginal bacterial infections | No | 12.5 | 87.5 | 72 | 6.388 | 0.011 |
| Yes | 100.0 | 0.0 | 1 | |||
| Acne | No | 11.6 | 88.4 | 69 | 4.717 | 0.030 |
| Yes | 50.0 | 50.0 | 4 | |||
| Pain during sex | No | 13.2 | 86.8 | 68 | 0.180 | 0.671 |
| Yes | 20.0 | 80.0 | 5 | |||
| Abdominal discomfort/pain | No | 14.5 | 85.5 | 69 | 0.672 | 0.412 |
| Yes | 0.0 | 100 | 4 | |||
| Breast tenderness/pain | No | 9.2 | 90.8 | 59 | 10.015 | 0.002 |
| Yes | 50.0 | 50.0 | 4 | |||
| Depression | No | 14.5 | 85.5 | 69 | 0.672 | 0.412 |
| Yes | 0.0 | 100.0 | 4 | |||
| Mood changes | No | 17.2 | 82.5 | 58 | 2.997 | 0.083 |
| Yes | 0.0 | 100.0 | 15 | |||
Association analysis between satisfaction with age, the impact of bleeding, pregnancy intention, counseling (Table 6), and side effects like less bleeding, no bleeding, or irregular bleeding (Table 7), were not statistically significant. The findings showed varied high satisfaction with the IUS according to age groupings but were not statistically associated at three months and 12 months post-uptake (Table 6). Satisfaction with the IUS at 12 months post-insertion was markedly higher among women who reported a positive impact of not bleeding from the use of the IUS, compared with those who reported a negative impact of not bleeding, although this was not statistically significant. With regards to pregnancy intention at 12 months post-up-take, women who reported having an intention to get pregnant in the next 3 years and above showed higher satisfaction (range between 82% – 100%) than those who reported having an intention to get pregnant at 2 years (77.8%), this association was not statistically significant.
Experience of satisfaction with the method at 12 months post-uptake was lower among women experiencing less bleeding (83.3%) than women not experiencing less bleeding (87.3%). Similarly, satisfaction was lower with the experience of no bleeding (80%) in contrast to the experience of bleeding (89.6%); these findings on the association between the bleeding changes and satisfaction, were, however, not statistically significant (p>0.05).
In our study, we found high continuation rates and satisfaction with the hormonal IUD among the users. During the use of contraception, adherence to the method is very important for the prevention of unintended pregnancies, as such the high continuation rate of the IUS contraceptive in our study is indicative of the method’s potential and acceptability to reduce the high risk of unwanted and unplanned pregnancies among women who genuinely need to continue contraception. Similar high continuation rates of the IUS in the first 12 months of use have been reported in previous studies12,19. The IUS has been reported to be highly effective and very tolerable, making the method very acceptable. Side effects are fewer and less serious, which is likely to influence the user's likelihood of satisfaction with and continuation of the method. As our study showed, the majority of the women were satisfied with the IUS, and this satisfaction is correlated to their lowered experience with side effects. Satisfaction from the IUS can also be related to its convenience (in the sense that once the method is inserted, nothing else regularly is needed to be done), as we found that this was among the major reasons the IUS was recommended to other potential FP users.
Experiencing no bleeding or less bleeding, which is a characteristic of the hormonal IUD, presented lower satisfaction (although not statistically significant) with the LNG IUS in our study, compared with other studies where women who experienced amenorrhea tend to appreciate more of this characteristic of the IUS method20. The reason for our findings may not be unconnected to the socio-cultural norms and beliefs that exist in the society about menstruation. Several studies have reported on the misconceptions and myths about menstruation, citing that some women believe menstruation to be a result of the body system discharging unwanted ‘bad blood’ from the body21,22. This assertion reasonably supports our findings for the lowered satisfaction with the method resulting from reduced or lack of menstrual bleeding. Conversely, we found that most women reporting a negative impact of non-bleeding due to the IUS were less satisfied. Our finding that older women (≥35 years) and women with more children (≥2 children) presented higher proportion to continue the use of the IUS, compared with their younger counterparts and women with fewer children (≤1 child), was not surprising. Several studies have also reported high continuation with LARC among women with more children23,24. Clinicians should encourage younger women and women with fewer children to use LARC as long as they need to delay pregnancies.
Adequate counseling and visiting a health provider for the uptake of health services has been linked with adherence to the use of the health service and of FP specifically25,26. This is important because patients/clients receive relevant information, access check-ups, and get informed about potential risks, fears, and misunderstandings that might exist and can affect the continuation of health services. We found in our study that more women who received counseling continued the use of the IUS than those who did not, this finding, however, was not statistically significant. Although women are free to discontinue the use of the method at any time, health providers offering support on the management of side effects and counseling on expectations of these side effects of the IUS may also have influenced the continuing use of the method that we observed. Previous studies have, however, reported provider bias in the uptake and use of FP methods to influence the choice of FP method27,28. Health providers, however, need to provide adequate information and counseling to clients to encourage optional use of an FP method to suit the client’s FP needs.
A strength of this study is that the data collection approach via phone interview can be thought to have guaranteed less respondent bias on the disclosure of private information about FP use and contraceptives. The limitation of this study, however, is the high attrition rate. Analysis showed some significant difference in the data between the age of participants between baseline and at midline, but not end line of the study, indicating that there was no serious potential attrition bias. Further, while the sampling for this study was widespread and done from 40 facilities across 17 states in Nigeria, given the small sample size and selection of facilities only from the private sector, caution should be taken on the generalization of the findings regarding users of the hormonal IUD in other regions in the country or across the entire country. Further studies are required on the reproductive and health profiles of the users of the IUS in Nigeria with larger sample size and control to improve retention rate. A comparative study with other LARC methods will also provide further insights into the full potential of the IUS method. Evidence is also required on the use of the method in Nigeria for the treatment of menorrhagia and dysmenorrhea, which is quoted as a characteristic of the IUS method, to fully take advantage of these benefits.
In conclusion, the hormonal IUD has positive potential as an acceptable intrauterine contraceptive given the high continuation rate and user satisfaction with the method. The hormonal IUD method presents few side effects which influenced continuation and satisfaction with the method and makes it highly recommended to other potential users of contraceptives. National action is, therefore, recommended for expanding the availability of the hormonal intrauterine system method in the public and private sector to reduce the unmet need for family planning in Nigeria.
Figshare: Continuation and user satisfaction of the levonorgestrel intrauterine system (LNG IUS) contraceptive in Nigeria, https://doi.org/10.6084/m9.figshare.12988313.v1129.
Figshare: Continuation and user satisfaction of the levonorgestrel intrauterine system (LNG IUS) contraceptive in Nigeria https://doi.org/10.6084/m9.figshare.12988313.v1129.
This project contains the following extended data:
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The authors would like to thank the women who agreed to take part in this study, and the health providers in the SFH Healthy Family Network social franchise who assisted in the recruitment of the women who participated in the study. We thank Kendal Danna and other members of the Population Service International - Washington D.C, for their expertise and contribution in supporting the implementation of the LNG IUS service delivery and the study in Nigeria with the Society for Family Health. Population Service International was also the co-principal investigator on the study in Nigeria.
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Contraception, abortion,
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: Personal fees from Bayer Sweden AB, for educational activities. Honorarium from Medsphere Corp USA, for expert opinions on LARC in Europe. I confirm that these potential conflicts of interest did not affect my ability to write an objective and unbiased review of the article.
Reviewer Expertise: long-acting reversible contraception, contraceptive counseling, emergency contraception, abortion
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 02 Feb 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)