Keywords
convalescent plasma, COVID-19, post-trial, follow-up, safety, elderly.
This article is included in the Coronavirus (COVID-19) collection.
convalescent plasma, COVID-19, post-trial, follow-up, safety, elderly.
COVID-19 convalescent plasma (CP) is one of the most studied pharmacological strategies during the pandemic1–4. In a recent double-blind, randomized, placebo-controlled trial we showed that early administration of high-titer CP against SARS-CoV-2 to mildly ill infected older adults was a safe therapeutic intervention and reduced the progression to COVID-19 severe respiratory disease (relative risk = 0.52; 95% confidence interval 0.29 to 0.94; p-value = 0.03) and a relative risk reduction of 48%5.
The most widely accepted theory on how CP acts in SARS-CoV-2 infection is by neutralizing antibodies inhibiting viral entry and amplification, mediating viral clearance, activating complement, enhancing the dependent cellular phagocytosis, and cytotoxicity, and providing anti-inflammatory cytokines6,7.
However, CP may also have a long-term negative effect by down-regulating the inflammatory response (a decrease in activated, effector, and memory CD4+ T cells, a decrease in activated and effector CD8+ T cells, and a decrease of naïve B cells)8, suppressing antibody formation9 and promoting autoantibodies against interferons10.
COVID-19 CP proved to be a safe intervention with <1% severe adverse events immediately after its administration2. Nevertheless, the long-term clinical effects of COVID-19 CP are to date understudied.
Our objective was to establish the long-term safety profile of COVID-19 CP and determine if its administration increases the risk for further respiratory infections in older adults.
Study population. The study by Libster et. al. included 160 mildly symptomatic participants infected with SARS-CoV-2 who were 75 years of age or older, or between 65 and 74 years of age with at least one coexisting comorbidity (hypertension, diabetes, obesity, chronic renal failure, cardiovascular disease, and COPD)5. Participants were randomized 1:1 to receive 250 ml of convalescent plasma (with an IgG titer > 1:1000 against SARS-CoV-2 spike protein) or 250 ml of placebo (0.9% normal saline) in less than 72 hours since the onset of symptoms. Next, they were strictly followed for 15 days to evaluate the development of severe respiratory disease5.
All patients included in the intention to treat (ITT) analysis of the original RCT study5 were invited to participate in a prospective cohort to assess for clinical differences over time among the CP and the placebo groups.
This follow-up study was part of the original protocol and was approved by the institutional review boards of each participating institution and the state of Buenos Aires (Comité de Ética Central de la Provincia de Buenos Aires, reference number: 2919–2129–2020, approved 15/5/2020; Clínica y Maternidad Suizo Argentina – Comité de Ética en Investigación, reference number: 1912, approved 19/6/2020; Swiss Medical Group, Clinica Olivos, approved 18/6/2020; Sanatorio Finochietto, approved 10/6/2020; Centro Gallego de Buenos Aires, Comité de Ética en Investigación Clínica, reference number: 1651/55/2020, approved 18/6/2020; Sanatorio Sagrado Corazon-OSECAC, approved 19/6/2020).
All participants or their legal guardians signed an informed consent and provided verbal consent during follow-up.
At the end of their participation in the RCT, all participants were instructed to communicate with the research team and report any adverse event, the onset of new symptoms, any confirmed diagnosis, and/or any visit to the health system. In addition, previously trained physicians using pre-designed questionnaires (available in section Data availability) phone-called all participants to assess for the variables and outcomes of interest at 3 and 6 months after randomization, and conducted a final contact between 7–10 months for data quality assurance. Data collection occurred between June 2020 and June 2021 in the city of Buenos Aires and Buenos Aires State, Argentina.
The primary endpoint of the follow-up study was the number of patients with clinically confirmed acute respiratory infections (ARIs) defined by the diagnosis of at least one of the following: pneumonia, upper respiratory infection, bronchitis, pharyngitis, or acute otitis media.
Secondary endpoints included all-cause mortality, time to first respiratory infection, SARS-CoV-2 confirmed re-infection, adverse events probably related to investigational product, and persistence of COVID-19 symptoms after initial infection.
Differences between participants were compared using the Student t-test and Chi-squared test, where appropriate. A p-value <0.05 was considered statistically significant. Time to first respiratory infection was assessed using the Kaplan-Meier method, and the log-rank test to assess for differences among groups (CP and placebo). We conducted a subgroup analysis by modified intention to treat (mITT) as in the original study excluding those randomized to CP but did finally not receive it5, and by those receiving a higher SARS-CoV-2 S IgG titer in donor plasma (using 1:3200 as a cutoff point). Stata/SE 13 package for IBM-PC (Stata Corp) was used for analysis and figure creation.
From the initial RCT that included a total of 160 patients in the ITT analysis (CP=80 and Placebo=80) and 154 in the mITT (CP=76 and Placebo=78), 153 were eligible to participate in the study (6 died during the original study and 1 withdrew consent). 142 patients were included in the follow-up study. 10 patients were lost to follow-up and 1 patient did not want to participate in the follow-up study, resulting in a total retention rate of 92.8% (CP=92.3% vs Placebo=93.3%) (Figure 1).
The mean age of included participants was 77.2 years (SD 8.6), and their median duration of follow-up was 10.4 months (IQR 1.63), with no differences among groups, with 10.3 months (IQR 1.68) and 10.42 months (IQR 1.6) for the CP and placebo groups respectively (t-value (df 140) =-0.35, p-value=0.724). No treatment switching was evidenced during the follow-up time. 89% of the participants (n=127) received a COVID-19 vaccine during the study with a median time to the first dose of 8.8 months (IQR 2.2).
14% of the participants (n=20) had a clinically confirmed ARI during the study. No differences were observed between groups (CP=11/72 and Placebo=9/70, x2 =0.17, p-value=0.678). The median time to first ARI was 8.63 months (IQR 7.13) for the CP group and 9.2 months (IQR 7.53) for the placebo. No statistically significant differences were observed in the probability of ARI-free survival between groups (log-rank test p-value=0.63) (Figure 2).
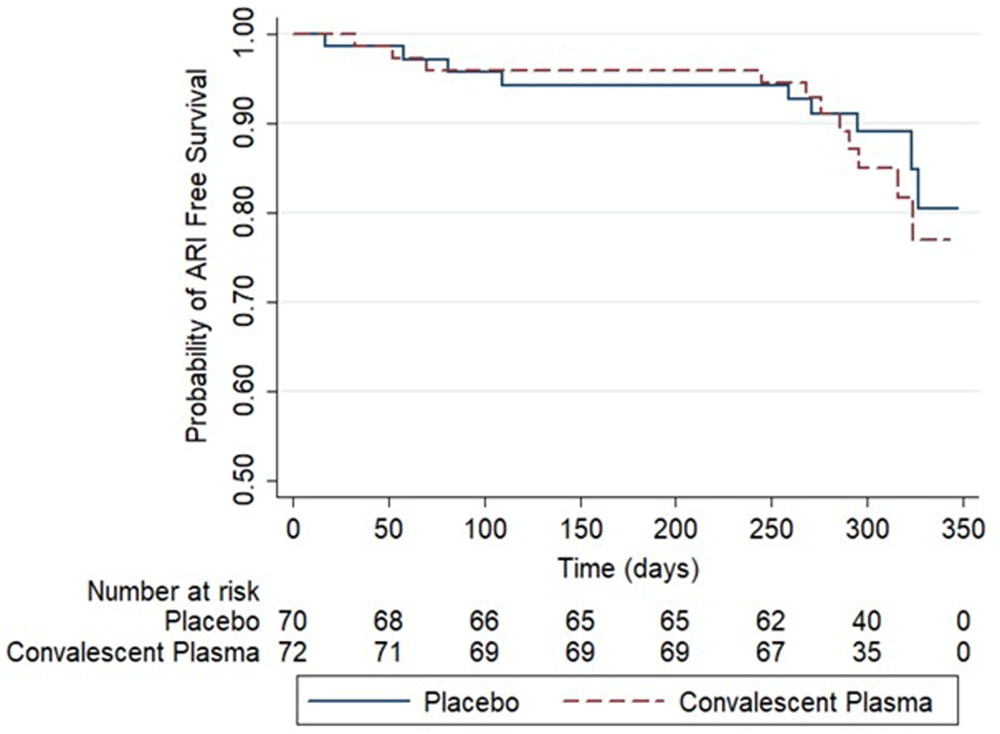
ARI = Acute Respiratory Infection. Placebo (solid line). COVID-19 convalescent plasma (dashed line).
CP and placebo patients did not present any adverse event related or probably related to the investigational products during this follow-up study. No differences emerged when comparing groups regarding total SARS-CoV-2 reinfections (CP=1/72 vs Placebo=1/70, x2 =0.0004, p-value=0.984), all-cause mortality (CP=5/72 vs Placebo=2/70, x2 =1.27, p-value=0.261), and in the number of patients with persistence of COVID-19 symptoms after the initial infection (CP=34/72 vs Placebo=30/70, x2 =0.27, p-value=0.601).
There were no statistically significant differences in the number of participants with ARI when comparing within the mITT subgroup (CP=10/69 and Placebo=9/68, x2 =0.045, p-value=0.831) or when comparing those who received SARS-CoV-2 S IgG titer in donor plasma ≥1:3200 vs. placebo (CP=3/34 and Placebo=9/70, x2 =0.36, p-value=0.546). All secondary endpoints remained stable with no difference by treatment in the subgroup analyses (data not shown).
In this post-trial follow-up study of an RCT of COVID-19 convalescent plasma in older adults, CP did not increase the risk for acute respiratory infections in the long-term follow-up. Furthermore, no differences were observed between groups (CP vs placebo) when assessing for time to first acute respiratory infection, adverse events, SARS-CoV-2 reinfections, all-cause mortality, and persistence of COVID-19 symptoms. In addition, all endpoints remained stable showing no difference in the subgroup analyses.
To our knowledge, this is the first post-trial follow-up after an RCT of COVID-19 CP assessing for long-term consequences of its administration. COVID-19 convalescent plasma remains a safe intervention with no clinical consequences in the long term.
A recent randomized clinical trial with control plasma by Sullivan et al. supports the hypothesis of our original study that COVID-19 CP should be administered early in the course of the disease and to those presenting mild symptoms11. In this study, COVID-19 CP presented a relative risk reduction of 54% in COVID-19 related hospitalization within 28 days after transfusion, which increased to 79.9% in those with ≤ 5 days of symptoms. However, no differences were found between groups when comparing patients already vaccinated11.
Our study adds evidence to the long-term safety of COVID-19 CP. This intervention might have now a lesser role as a strategy against SARS-CoV-2, however, our study supports an early and coordinated evaluation of convalescent plasma use in future pandemics.
figshare: Post-trial follow-up after a randomized clinical trial of COVID-19 convalescent plasma - Stata Dataset. https://doi.org/10.6084/m9.figshare.20311152.v112
This project contains the raw data file.
figshare: Post-trial follow-up after a randomized clinical trial of COVID-19 convalescent plasma - Stata do-file. https://doi.org/10.6084/m9.figshare.2031115513
This project contains the Stata do-file.
figshare: Codebook. https://doi.org/10.6084/m9.figshare.20349534.v114
This project contains the database codebook.
figshare: Data collection instruments & questionnaires – English. https://doi.org/10.6084/m9.figshare.20402136.v115
This project contains the data collection instruments and questionnaires in English.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Libster R, Pérez Marc G, Wappner D, Coviello S, et al.: Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. New England Journal of Medicine. 2021; 384 (7): 610-618 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Public Health, Epidemiology, Clinical Research, Pediatrics, Pediatric Critical Care
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Public Health, Epidemiology, Cardiology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Aug 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)