Keywords
pneumococcal vaccine, immunization, digital health, mHealth,
pneumococcal vaccine, immunization, digital health, mHealth,
Pneumococcal conjugate vaccines (PCVs) have been introduced in many parts of the world. PCVs prevent infection by pneumococcus, a major cause of severe bacterial pneumonia globally. The introduction of PCVs into lower and middle income countries has been a major priority for national governments and international health organizations. Globally, India has the highest number of under 5 deaths caused by pneumococcus. Given the high burden of pneumococcal disease in India and the size of the population1, the introduction of PCVs has the potential to have a major impact2,3. Starting in May 2017, several states and high-risk districts in India introduced PCVs into the routine childhood immunization schedule with two primary doses and a booster dose (2+1 schedule).
As PCVs are incorporated into the immunization schedule in India, it is critical to ensure that they are being used optimally. Key performance indicators for PCV include: the percentage of the eligible population receiving 1, 2 and 3 doses of the vaccine respectively (coverage), the percentage of children completing their vaccination series (completion), and the percentage of children receiving these doses according to the vaccine schedule (timeliness)4. However, monitoring this information on a paper-based system has limitations. Prior research has shown that paper-based tracking is often incomplete and lacks accountable, timely, and actionable data5. Digital community health platforms have the potential to fill this gap, even in hard-to-reach rural populations4.
In this study, we use data from Khushi Baby6, a novel digital health platform that captures individual-level data on maternal and child health care encounters at government-run Village Health and Nutrition Day (VHND) camps in five predominantly rural administrative blocks in the Udaipur district. The analysis of these data provides village-level resolution of the roll-out of PCVs in a high-priority rural district in northern India.
At the monthly health camps, caregivers of infants are registered with the Khushi Baby mobile application. The medical history (including age, location, vaccination history, weight-for-age, and breastfeeding status) and biometric template are stored in a Near Field Communication (NFC) chip, embedded in a culturally-relevant amulet or health card. When the caregiver and child return to the follow-up VHND camp, the NFC chip is scanned by the health worker’s mobile device. To authenticate the beneficiary, the primary caregiver’s live thumbprint is compared against the biometric template stored at the time of registration. Now, the health worker can instantly view the beneficiary’s comprehensive medical history. The health worker’s mobile device also captures the timestamp and GPS location of the health camp. Together, these mechanisms help ensure that the health worker attended the camp and met the reported child, especially in rural areas where internet connectivity, data quality, and supervision are inconsistent. The NFC chip further provides a digitally auditable trail of the health interaction. Children residing in five administrative blocks in the district of Udaipur, serviced by government outreach camps, are present in the database. For the study analyses, we used individual-level data detailing the date of birth, administrative region of residence, whether the beneficiaries were offered and accepted vaccination with PCV, and the date of administration of the first, second, and booster doses.
Data on vaccine uptake by age were analyzed, using software package R 3.6.3, as a survival function, where the ‘time’ was the number of days since birth, and an event was the receipt of the vaccine. A child was censored if they did not receive the vaccine as of October 20, 2020 or when they turned 12 months of age. The system did not record receipt of PCVs received after 12 months of age. Separate analyses were conducted looking at receipt of the 1st dose, the 2nd dose, or the booster dose. Data were also disaggregated by block to evaluate geographic variations in uptake.
In separate analyses, data were also aggregated by month of birth to evaluate the percentage of children in each birth cohort that had received 1, 2 or 3 doses as of October 20, 2020. Aggregating by birth month can help to identify seasonal variations as well as to track uptake based on eligibility. Additionally, we also aggregated by gender to look for an effect of gender bias on vaccine uptake.
Vaccine timeliness was evaluated as the proportion of vaccinated children who received their dose within 1 month or 2 months of the recommended age.
As a comparison, we analyzed receipt of pentavalent vaccine (scheduled for 6, 10, and 14 weeks) and the first dose of measles vaccine (scheduled for 9 months). The pentavalent vaccine can be compared with the receipt of the primary doses of PCVs, while the measles vaccine can be compared with the booster dose of PCV.
The Khushi Baby platform captured data on 18,122 children born between February 9, 2017 and October 11, 2020 in Udaipur District, India (Figure 1). Of these, there were 10,202 children born on or after March 1, 2018, which includes the first group of children who received PCVs at a high rate. The children in the database reside in 5 administrative regions, with 1239–4965 children per region (Table 1), with Gogunda performing the best and Jhadol performing the poorest with respect to PCV completion. The Khushi Baby platform is deployed in villages where government camps service approximately 25% of the children born in the catchment area. The rate of reporting through the Khushi Baby platform varies between different Auxiliary Nurse Midwives (ANMs). We can assume each ANM on average reports half of the patients who turn up to a given maternal child health camp.
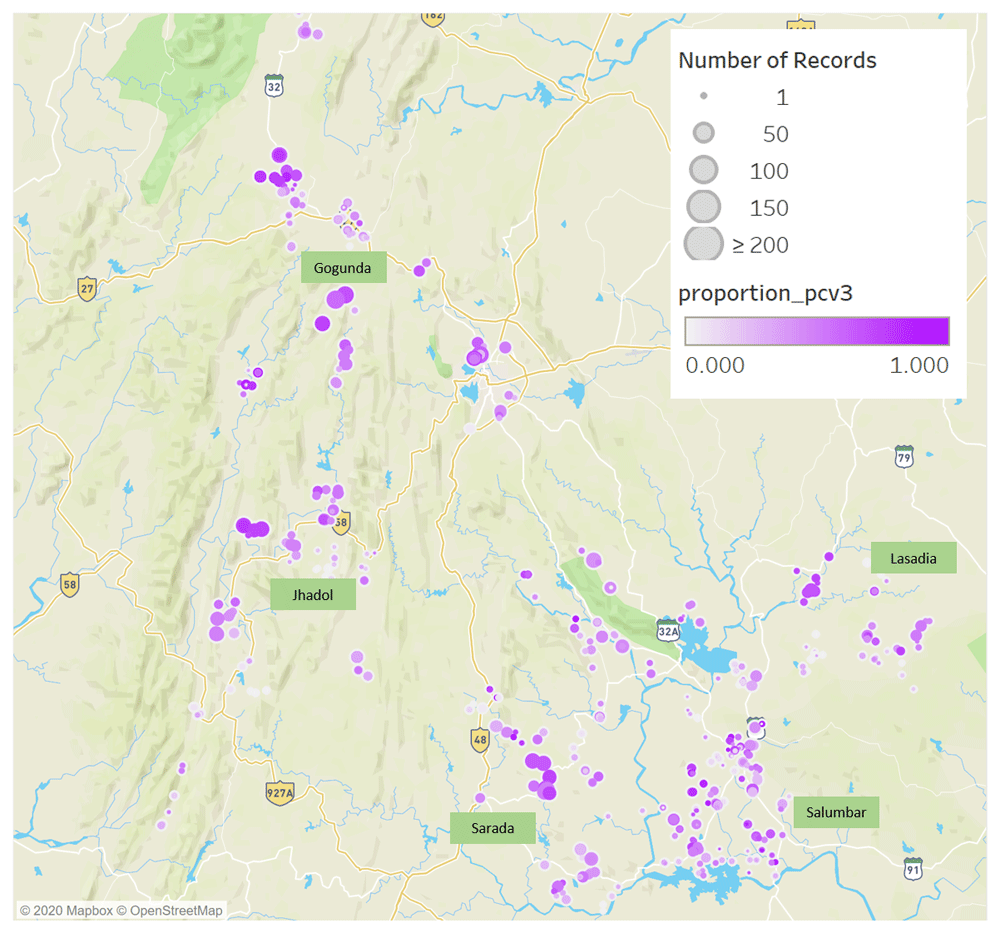
Visualization made using Tableau Software 2019.4.
Initiation of vaccination increased for children born in late 2017 and early 2018 and stabilized for children born on or after March 2018 (Extended data: Figure S17). Almost all of the children (99%) captured by the system and born after March 1, 2018 received the first dose of PCV13 (Figure 2). 80% of the children received the 2nd dose of the vaccine. 56% of children received the booster dose by 12 months of age (Figure 2). There was considerable variability in vaccine uptake between the five blocks. (Figure 3). All five of the blocks had similarly high levels of uptake of the first dose of PCV, with >96% uptake. However, uptake of the 2nd dose ranged from 69% to 90%, and uptake of the booster dose ranged from 44% to 76%. Children born more recently had lower uptake of the 2nd and 3rd dose, leading to lower apparent PCV completion rates (Extended data: Figure S17). Finally, there was no significant gender effect on vaccine uptake. Although uptake in the winter months was lower for PCV2 and 3 doses in 2018, a clear seasonal trend was not clearly discernible over multiple years.

Only children born after March 1, 2018 are included.
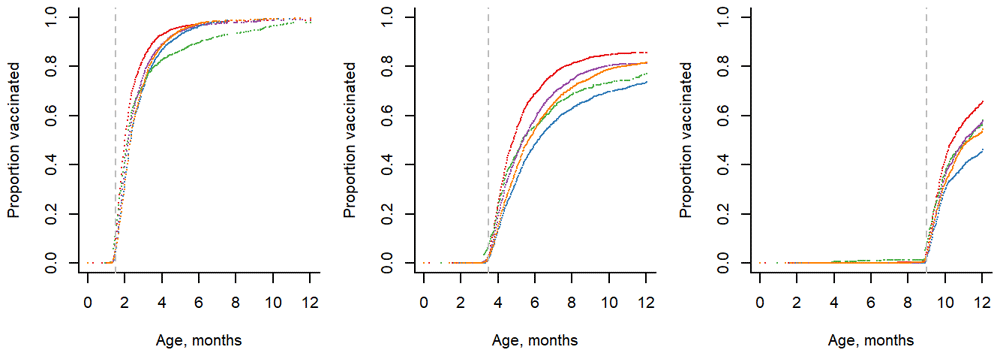
Gogunda (red), Salumbar (purple), Lasadia (Green), Jhadol (blue), Sarada (orange). Children born after March 1, 2018 were included. For all doses, Gogunda performed best.
These uptake values were comparable with pentavalent vaccines (scheduled for 6, 10, and 14 weeks) and the first dose of measles vaccine (9 months). By 12 months of age, 99%, 89%, 79% received the first, second, or third doses of pentavalent vaccine, respectively. 60% received the measles dose, recommended at 9 months of age. By 2 years of age, the uptake of the measles dose increased to 69% (Extended data: Figures S2, S37).
Vaccine timeliness can be evaluated with these data as well. Of those children vaccinated, 64% received their first dose of PCV13 within a month of the recommended age (6 weeks), and 85% of the children received it within 2 months of the recommended age (Figure 4). The timeliness of the 2nd dose was lower, with 40% and 64% receiving the dose within 1 or 2 months, of the recommended date, respectively. The timeliness of the booster dose can’t be fully evaluated because data were only available for 3 months after the recommended date, but most children who were vaccinated by age 12 months were vaccinated within 1–2 months of the recommended age (Figure 4).
These analyses provide important information on the extent to which PCV is reaching infants in rural India. A large proportion of the children captured in the database received their two primary doses of the vaccine. However, receipt of the booster dose within 3 months of the recommended date was suboptimal in most of the regions, and this trend mirrors findings seen for the measles vaccine with the same scheduled due date. Potential demand-side reasons for this dropoff might include a lack of health awareness among primary caregivers and a deviation in the monthly pattern of vaccinations within the first four months.
The majority of the benefit of PCVs is attributed to reductions in transmission (indirect protection)8 . The booster dose is particularly important in reducing the prevalence of vaccine-targeted serotypes among the healthy children who carry pneumococcus and in maintaining long-lasting memory responses9,10. Therefore, improving uptake and timeliness of the booster dose in this population should be a priority.
The ability to evaluate variations in uptake at a local scale is enabled by the Khushi Baby system. We found substantial variations between the regions in the receipt of the 2nd and 3rd doses, and this information could be used to prioritize efforts to increase uptake in target populations. A possible decline in uptake during the winter months may be attributed to the overlap with the kharif harvest and festive season. Future work will explore how automated, targeted voice call reminders in the local dialect, already in place for other vaccines within the Khushi Baby system, could be used to decrease the drop out in PCV in combination with boots-on-the-ground campaigns.
Disruptions related to the COVID-19 pandemic influenced vaccination rates in the first two quarters of 2020. This is evident from the decline in children born near June 2019 receiving the booster dose of PCV (Extended data: Figure S17).There was a nationwide lockdown from March 19, 2020 to June 30 2020, which resulted in suspension of vaccination camps. Normal activity of VHND camps resumed by July 2020. If children ultimately received their booster dose but received it after 12 months of age, we would not have captured it in our database.
There are important limitations to these data. First, we only capture 30% of the children in this region, who might not reflect the vaccination coverage in the broader population. The actual immunization rates could be lower if our sample is biased towards people who are seeking healthcare. Likewise, the uptake of the first dose is likely biased upwards because these are children who have initiated contact with the healthcare system at a vaccination camp. Second, when there is no record of a child receiving subsequent doses, it is not clear if this child truly missed their vaccine, received the vaccine elsewhere and was not recorded, or moved out of the area (lost to follow up). Nonetheless, these data are of a higher quality and reliability, and are more complete than alternative data sources for this population5.
In conclusion, we have demonstrated the ability of a digital health platform to track the introduction of a high-priority vaccine into a rural area of India. These data and analyses highlight areas where public health practitioners could target interventions to improve the implementation of the vaccine program.
These data are comprised of individual health records, including dates of medical visits and birth date. Therefore, only aggregate data can be shared. Data can be requested from the Khushi Baby data committee via email to admin@khushibaby.org
Analysis code is available from: https://github.com/weinbergerlab/kb-pcv
Archived analysis code as at time of publication: http://doi.org/http://doi.org/10.5281/zenodo.426532811
License: Apache-2.0 License
Figshare: PCV Vaccine Uptake in Rajasthan Extended Data, https://doi.org/10.6084/m9.figshare.13573877.v17
This project contains the following extended data:
- Supplementary Figure 1. Percent of children who have received 1 dose (black), 2 doses (blue) or 3 doses (red) of PCV by 12 months of age as of October 20, 2020, by month of birth.
- Supplementary Figure 2. Percent of children receiving 1, 2, or 3 doses of pentavalent vaccine (dose=1,2,3), or the first measles dose (dose=4) by age in months.
- Supplementary Figure 3. Percent of children who have received 1 dose (black), 2 doses (blue) or 3 doses (red) of pentavalent vaccine; or 1 dose of measles vaccine (gray) as of October 20, 2020, by month of birth.
License: Apache-2.0 License
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious disease epidemiology, immunization
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical pediatric infectious diseases, child health public health programming, implementation science
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Jan 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)