Keywords
COVID 19, SARS-CoV-2 , PPE, Cloth mask, N95, Surgical mask
This article is included in the Coronavirus (COVID-19) collection.
COVID 19, SARS-CoV-2 , PPE, Cloth mask, N95, Surgical mask
The coronavirus disease 2019 (COVID-19) pandemic’s first cases were reported in China in January of 2020 (World Health Organization, 2021). As of June 15, 2021, the World Health Organization has reported a total of 175,987,176 confirmed cases globally and 3,811,564 deaths. Many communities across the globe have shut down schools, businesses and travel in attempts to decrease the number of new infections. The virus can cause a range of symptoms including cough, shortness of breath or difficulty breathing, fever, chills, repeated shaking with chills, muscle pain, headache, sore throat, new loss of taste or smell and severe acute respiratory failure (Centers for Disease Control and Prevention, 2020). In addition, there is a portion of the population who are asymptomatic with the infection or infectious prior to any symptoms (Li et al., 2020; McMichael et al., 2020; Pan et al., 2020; Rothe et al., 2020). The ability for this novel strand of the virus to spread through respiratory secretions and other airborne media, even when carriers are asymptomatic or have mild symptoms, has made it difficult to contain.
There are multiple components involved in transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19. This inhaled pathogen is transmitted from person to person by way of expelled airborne particulate or ‘aerosols’ excreted from respiratory secretions through coughing, sneezing, singing etc. It may also be transmitted through contact with respiratory secretions on surfaces, although it is estimated that droplet and inhalation transmission routes outweigh contact transmission. (Asadi et al., 2020; Jones, 2020) Aerosols from infected persons pose an inhalation threat even at considerable distances and in enclosed spaces, particularly if the area is poorly ventilated. Expelled aerosol may carry further than the recommended six feet of distancing with a strong cough or sneeze (Jones & Brosseau, 2015; Verma et al., 2020).
The size of the SARS-CoV-2 virus is documented with a diameter ranging from 0.06-1.4 µm with spikes extending 0.009-0.012 µm (Zhu et al., 2020). Respiratory droplets can be of various sizes and are commonly classified as aerosols (<5 µm) and droplets (greater than 5 µm) (Konda et al., 2020). A systematic review of 26 studies reporting particle sizes generated from breathing, coughing, sneezing and talking, demonstrated that healthy individuals generate particles between 0.01 and 500 µm and individuals with infections produce particles between 0.05 and 500 µm (Gralton et al., 2011). There is also evidence that sneezing can expel sheet-like layers of respiratory fluids which break apart into various sized particles and fluid which falls quickly (Scharfman et al., 2016). This indicates that expelled particles carrying pathogens, such as SARS-CoV-2 likely do not exclusively disperse by airborne or droplet transmission but by both methods simultaneously (Gralton et al., 2011).
Apart from COVID-19, respiratory droplets are the primary means of transmission for other illnesses including the common cold, influenza, tuberculosis, SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome) (Verma et al., 2020). Inhaled droplets and aerosol particles have different sites of deposition in the recipient. Large inhaled droplets are deposited on the face or in the upper regions of the respiratory tract. In contrast, inhaled droplets and viral particles <5.4 µm can penetrate to the depths of the lungs, where they may then be deposited in the bronchial tubes and alveoli. Due to the airborne nature by which SARS-CoV-2 virus is transmitted, it is highly contagious and has potential for rapid spread, morbidly, and mortality.
Previous studies have demonstrated some benefits of home-made masks, however their filtration was not equivalent to surgical masks or other commercially available respirators (Asadi et al., 2020; Davies et al., 2013; Konda et al., 2020; van der Sande et al:., 2008; Verma et al., 2020). Therefore, we sought to explore if adding a filter to a cloth mask could improve the performance. This in vitro study was designed to evaluate mask efficiency and prevention of potentially infectious airborne particles between no mask, cloth mask (with/without filter), surgical mask, and N95 respirators to prevent inhaled airborne exposure of ‘coarse’ aerosols and ‘fine’ water particles. The study sought to test the hypotheses that there is no difference in the filtration of aerosol particles and water vapor delivered to an adult 3D printed airway model in a spontaneously breathing test lung between cloth mask with/without a filter, s surgical mask, and a N95 respirator.
The study was performed at the Seattle Children’s Research Institute, Seattle, WA from April 2020- Oct. 2020. Studies in vitro were carefully designed to evaluate protective attributes based on the efficiency and permeability between simple cloth face masks, commonly worn in the community, and those applied in hospital settings. We also measured efficiency with the addition of a filter to the cloth mask.
Initially, we evaluated efficiency on prevention of inhaled ‘fine’ particles (0.03–0.05 µm) by assessing mask permeability of water vapor generated by a humidifier. These water vapor particles, also capable of transmitting airborne viruses, are generated in the exhaled breath of infected individuals while talking and present a significant risk for infection. We then characterized albuterol particle size generated by a nebulizer with a particle impactor. Lastly, we applied the nebulizer to a spontaneously breathing 3D printed airway affixed to an adult lung model and quantified particle deposition to the masks and filters (when applicable) and at the level of the face, airway model, and lung model filter. For this study, we considered nebulized aerosols as “coarse” particles within the range of particle sizes known to generate infectious airborne droplets generated through coughing or when performing aerosol generating procedures (AGPs) by caregivers in clinical settings.
Figure 1 A–H show the different mask and filters used with each of the experimental conditions. The cotton or “cloth” facemasks, unisex fashion stretch lightweight cotton covering face and mouth (Getien Biotech, Inc; Nanjing, China), were chosen as they had elastic ear loops, a filter pocket and nose wire that allowed for fit adjustment (Figure 1 C, D, E). An N95 respirator (Figure 1A) (3M™; St. Paul, Minneapolis) and surgical mask (Figure 1B) (Wujin Economic Development Zone; Changzhou, Jiangsu) are commonly used in the hospital setting for respiratory precautions were also used.

The different masks used in all of the experimental conditions are shown, with N95 (A), surgical mask (B), cloth mask posterior (C), and anterior (D), with filter pocket (E). Filters used with cloth masks included PM2.5(F) MERV 15 (G) and Swiffer (H).
Prior to testing, masks and mask filters were preconditioned using a vacuum warmer (Labconco 7982010 CentriVap Aqueous System, 115V, 60Hz) at 40°C for 30 minutes. Three commercially available filter materials were used including PM2.5 filters (Getien Biotech, Inc; Nanjing, China) (Figure 1F), MERV 15 (Capital air filter; Raleigh, NC) (Figure 1G), and unscented Swiffer™ Sweeper Dry™ sweeping cloth (Procter & Gamble; Cincinnati, OH) (Figure 1H). Both the MERV 15 filter and the Swiffer™ dry cloth were cut to the same dimensions as the PM2.5 filters (12cm x 8cm) (see Figure 1F, G, H).
Both phases of testing were conducted by evaluating 5 separate runs at each of the following conditions: 1) no mask; 2) cloth mask; 3) cloth mask with Swiffer™ filter; 4) cloth mask with MERV 15 filter; 4) cloth mask with PM2.5 filter and 5) surgical mask. Owing to a critical shortage of N95 masks in our region, at the time of testing, we were only able to test 3 each of the N95 respirator. For the fine particle testing we repeated testing on two of the masks after drying for a total of 5 runs (N=5). These same three masks were used for course particle testing for a total of 3 runs (n=3).
The first phase of testing evaluated mask efficiency based on ‘permeability’ of fine water vapor particles. A 3D printed face and nasopharyngeal model was used. This airway was replicated from the Computed Tomography (CT) scans of a 17-year-old male’s head (printed with PLA black material on Crealty CR 10 printer with embedded nasa airway printed with FormLabs Clear V4 resin on the Form 2 printer). Spontaneous breathing was simulated using a Harvard Large Animal Ventilator (Model #613, Harvard Apparatus; Holliston, Massachusetts). The model simulated normal breathing through different mask conditions (Figure 2A).

(A) The experimental set-up of the Harvard Apparatus use to emulate spontaneous breathing via 3D- printed realistic adult airway model. (B) Assessment of fine particle efficiency was characterized by measuring time to reach 95% humidity levels acquired from within the simulated trachea during spontaneous breathing. A valved, aerosol mask allowed egress of exhaled water vapor particles to atmosphere to prevent saturation of masks/filters and limit measurement to incoming water vapor particles. (C) Direct application of aerosol applied to the face with a cloth mask. Aerosol was eluted from upper airways, and lung model filter placed in filter housing following nebulization of 10 mg albuterol and quantified using spectrophotometry.
The adult test lung was configured with pre-set tidal volume of 700 mL, respiratory rate of 20 breaths/min, and inspiratory: expiratory (I:E) ratio of 1:1 with half sinusoidal inspiratory and expiratory flow profile to mimic normal spontaneous breathing. (Li et al., 2019) All values were monitored prior to testing with the TSI 5200 analyzer. A traceable hygrometer (Model #4185, Fisher Scientific, Pittsburg, Penn) was placed within the simulated trachea in series between the 3D printed airway and the lung model to measure relative tracheal humidity (RH). Increases in tracheal RH level beyond the ambient value was indicative of ultra-fine water vapor permeating beyond the different masks and filters. A pediatric Monaghan AeroChamber Plus Z STAT valved mask (Monaghan Medical; Plattsburgh, NY) incorporates a series of low resistance one-way valves that allows decoupling of inspiratory and expiratory flow paths. This prevented saturation of the hygrometer but also limited changes in RH and penetration of incoming fine particles to inhalation without rebreathing exhaled particles. Following preconditioning of the mask and filter as applicable, the valved, aerosol mask was secured on the 3D model and masks using rubber bands. A Fisher and Paykel (MR 850, Fisher & Paykel; Panmure, Auckland) heated humidifier was preset at 37°C in the invasive mode and applied to 20 L/min air via a heated-wire circuit (see Figure 2B). RH value of 99–100% was confirmed at the AeroChamber mask opening using a hygrometer. Following ambient RH measurement, humidified air was administered directly to the airway with each mask/filter. The masks efficiency in filtering fine airborne particles was determined based on time (seconds) to reach 95% relative humidity within the trachea. A new mask/filter was used for each of the 5 runs with the exception of the N95 respirators. Three of the respirators were pre-conditioned again in the dryer and re-tested for a total of 5 runs (n=5).
The first step for the aerosol testing was to characterize the course particles size emitted from a nebulizer. Coarse aerosol particles were generated with a vibrating mesh (VM) nebulizer (Aerogen Solo, Aerogen Ltd., Galway, Ireland), with a residual drug volume < 0.1 mL. (Li et al., 2019) A high-performance, multistage, next generation impactor (Model #NGI-0670, NGI, TSI Incorporated, Shoreview, MN) was used to quantify the distribution of coarse aerosol droplet size produced by the VM nebulizer and classify into respirable size fractions. The NGI uses eight different particle trays to collect aerosol droplets. The vacuum flow was adjusted with a frit resistor (S/N 511197-9, Cole Palmer, Vernon Hills, IL) at 15 L/min and confirmed with a TSI 5200 Flow and Pressure Analyzer (TSI Industries, Shoreview, MN). A standard leak test was performed per manufacturer’s specifications before each test. 10 mg of aqueous albuterol was aerosolized in continuous output mode. Following nebulization, aerosol droplets were eluted by instilling 10mL of hydrochloric acid in each plate and then withdrawing the solution from each individual stage. The mass was quantified using ultraviolet-visible spectrometry (UV-Vis) (Molecular Devices SpectraMax M3; San Jose, CA). The albuterol sulfate was measured by UV-Vis spectrometry at its local spectral maximum of approximately 276 nm. Using the Spectra Max M3 (Molecular Devices; San Jose, CA). Samples were pipetted into 96 well plates with UV transparent flat bottom, (Thermo Scientific, or equivalent). Albuterol sulfate powder (USP Reference Standard, Cat #1012633) was used to make standard concentrations for comparison of unknown samples. A calibration curve was created using 500 µ/ml, 200 µ/ml, 100 µ/ml, 50 µ/ml, 25 µ/ml, and 12.5 µ/ml known concentrations of albuterol. Three readings from each of these know concentration samples were plotted and analyzed by the Spectra Max M3 software. The standard curve was then be used to determine the concentrations of test samples from the absorptance measurements. This was repeated for a total of 5 runs (n=5). We then calculated median mass aerodynamic diameter (MMAD), geometric standard deviation (GSD) using a log-probit equation, fine particle fraction (FPF, %) based on proportional mass of respirable particles (MMAD <5.4 µm) and total drug mass delivered to the impactor stages.
Following the characterization of aerosols emitted from the nebulizers, we evaluated filtration efficiency of coarse aerosols between the different mask and no mask conditions using a similar experimental set-up as the previous study and is shown in Figure 2A. A low dead-space filter housing containing a low resistance medication filter (PARI Filter; Starnberg, Germany), was placed between the lung model and simulated trachea to collect inspired aerosol just prior to entering the lung model. 10 mg of aqueous albuterol ulfate (5mg/mL) was administered through the VM nebulizer with an operational flow of compressed at 3 L/min. The nebulizer was attached directly to the 3D printed face model or overprotective masks using a vented aerosol mask (see Figure 2C).
Following nebulization, the lung filter was removed and the 3D printed face was wiped with a PARI filter. The 3D printed model airway was eluted with 20mL of hydrochloric acid (0.1 N). PARI filters, mask and mask filters (when applicable) were placed in a sealing plastic bag and eluted with 20 mL of HCL. They were manually agitated for 3 minutes. All eluents were filtered through a 5-micron filter needle to remove debris. Recovered samples were then quantified for absolute albuterol mass (µg) using UV-Vis spectrophotometry (Molecular Devices SpectraMax M3; San Jose, CA). The albuterol sulfate was measured by UV-vis spectrophotometry at its local spectral maximum of approximately 276 nm. Using the Spectra Max M3 (Molecular Devices; San Jose, CA). Samples were be pipetted into 96 well plates with UV transparent flat bottom, (Thermo Scientific, or equivalent). Albuterol sulfate powder (USP Reference Standard, Cat #1012633) was used to make standard concentrations for comparison of unknown samples. A calibration curve was created using 500 µ/ml, 200 µ/ml, 100 µ/ml, 50 µ/ml, 25 µ/ml, and 12.5 µ/ml known concentrations of albuterol. Three readings from each of these know concentration samples were plotted and analyzed by the Spectra Max M3 software. The standard curve was then be used to determine the concentrations of test samples from the absorptance measurements. A new mask (with or without filter) was used for a total of 5 runs (n=5). Each of the 3 N95 respirators were tested once for a total of 3 runs (n=3).
For these testing purposes, we reported coarse aerosol as the cumulative deposited aerosol dose (%) because patients are most likely to become infected by touching the face or when infectious droplets are inhaled directly into the naso-oral airway airways, bronchial tubes or lungs. As such, cumulative deposited aerosol dose (%) included the sum of albuterol mass deposited on face, airway, and lung as referenced to the nominal dose placed into the nebulizer. We also included descriptive measurements for mask and filter deposition.
Fine particle permeability based on time to 95% humidity was calculated in seconds and assessed for normality using histograms and QQ-plots and summarized using means and standard deviations for each mask/filter type. ANOVAs were used to compare the cumulative deposited dose (%) as well as the mean amount of time, in seconds, until the mask/filter reached 95% humidity between each mask/filter. The sum of cumulative albuterol particles deposited and recovered from face, airway, and lung was calculated and assessed for normality using histograms and QQ-plots. The cumulative mean value was referenced to the nominal dose placed into the nebulizer (10 mg) and at each individual location (face, airway, lung, filter, and mask) and expressed as means and standard deviations for cumulative deposited aerosol dose (%) for each condition. A Tukey’s post-hoc analysis was used to assess specific differences between each mask/filter type. A p-value < 0.05 was considered statistically significant. SAS 9.4 (Cary, NC) was used for all analyses. R is an open access alternative that could be used for the analysis. The deposited dose (%) at each individual location (face, airway, lung, filter, and mask) was not tested for differences but were included as descriptive data.
Estimates from previous pilot runs were used to estimate a standard deviation of 92 for total drug deposition. With 7 conditions of 5 runs each, this estimates a standard deviation of means of 64. An overall difference of 183 between mask/filter types was able to be detected using an alpha of 0.05 and 80% power. Post-hoc analyses could detect a difference of 232 between each mask/filter type with 80% power using a Bonferroni correction and an alpha of 0.007.
The results from the fine particle testing can be found in Figure 3. Based on time to 95% RH, permeability with the PM2.5, MERV 15, Swiffer™, and N95 was lower than no mask (Ringer, 2021). The cloth mask and surgical mask alone did not demonstrate significant (P<0.05) filtration of water vapor when compared to no mask condition. The cloth mask with Swiffer™ filter performed as well as the N95 respirator with filtration of the fine water vapor particles.
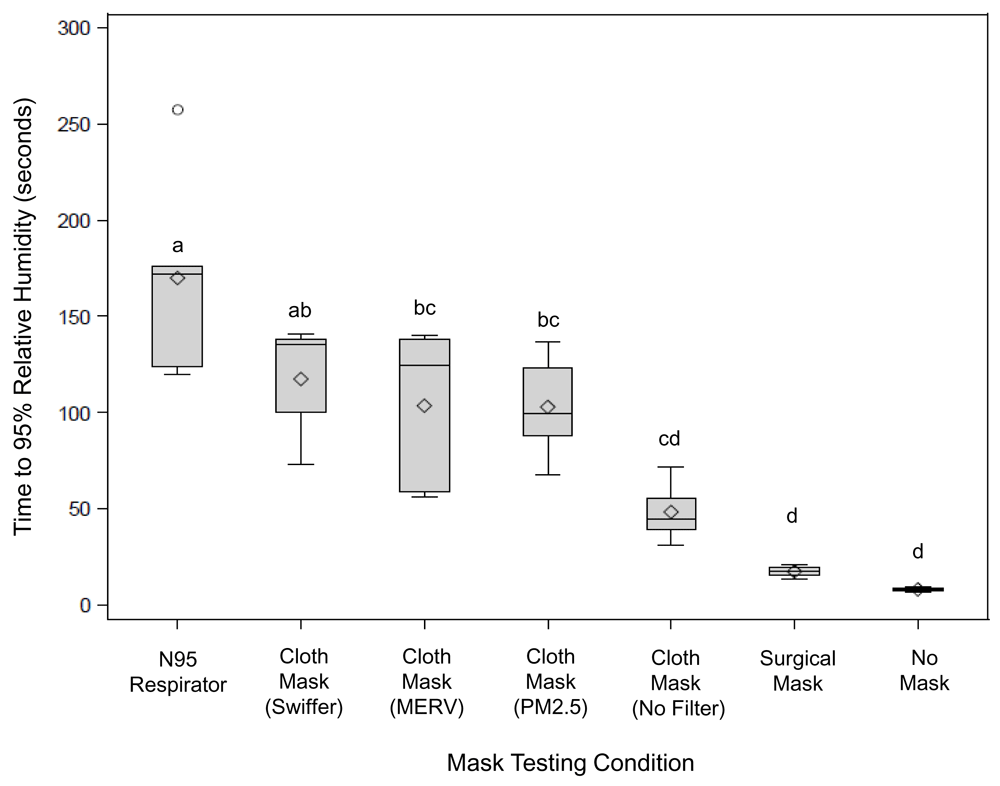
Data shown represent the time to 95% relative humidity (seconds), based on tracheal relative humidity levels, following exposure of the 3D airway to humidification with and without different mask options. Data are mean ± SD (standard deviation) with n=5 runs with each configuration. Data were analyzed with one-way ANOVA (analysis of variance), with Tukey test post hoc comparisons between testing conditions. Means with the same letter are not significantly different, P<0.05.
Nebulized coarse aerosol albuterol particle sizes ranged from 0.54 to 11.72 µm with MMAD of 4.87 ± 2.18 µm (Figure 4). The particle size fractions (%) were based on the total mass (µg) of albuterol recovered from the individual impactor stages with 43, 27, and 30% for particles >5.4, 3.2- 5.4 and <3.2 µm, respectively (n=5).

The particles generated by the Aerogen. Aeroneb (n=5) were characterized using an aerosol impactor prior to testing with masks. Particle sizes ranged from 0.54 to 11.72 µm with median mass aerodynamic diameter (MMAD) 4.87±2.18 µm. The particle size fractions (%) were based on the total mass (µg) of albuterol recovered from the individual impactor stages with 43, 27, and 30% particle for particles >5.4, 3.2- 5.4 and <3.2 µm, respectively (n=5).
Data shown in Figure 5 are the sum of total cumulative mass of albuterol particles recovered from the face, airway, and lung with and without different masks. These data were then referenced to the nominal albuterol dose (10 mg) placed into the nebulizer and shown as delivered dose %. Data are mean ± SD with n=5 runs with each configuration and n=3 for N95. Data were analyzed with one-way ANOVA, with Tukey test post hoc comparisons between testing conditions. Testing conditions with the same letter on Figure 5 have means that are not significantly different, P<0.05.

Data shown are the sum of total cumulative mass of albuterol particles recovered from the face, airway, and lung with and without different masks. These data were then referenced to the nominal albuterol dose (10 mg) placed into the nebulizer and shown as delivered dose %. Data are mean ± SD (standard deviation) with n=5 runs with each configuration and n=3 for N95. Data were analyzed with one-way ANOVA (analysis of variance), with Tukey test post hoc comparisons between testing conditions. Means with the same letter are not significantly different, P<0.05.
There were differences in the cumulative deposited dose (%) between different testing conditions (P<0.05). The no mask condition was the least efficient at preventing coarse particles from depositing on the face, airways, and lungs when compared to all of the different mask testing conditions. Although the cloth mask alone showed greater efficiency with a nearly 5-fold reduction in coarse particle transmission than the no mask condition, the addition of a filter (Swiffer™, PM2.5, or MERV 15) demonstrated greater filtration efficiency based on cumulative deposited dose (%). Surgical masks showed lower cumulative deposited dose than did no mask, cloth only, and cloth with Swiffer™ filter. The N95, prevented particle transmission with lower deposition than all the testing conditions except surgical mask and cloth mask with MERV 15 filter where performance was not different. Figure 6 shows descriptive data on the deposition of aerosol droplets measured in filters, masks, face, airways and lungs. The drug mostly deposited in the mask/N95 respirator with negligible delivery to the face, airway, and lung filter.
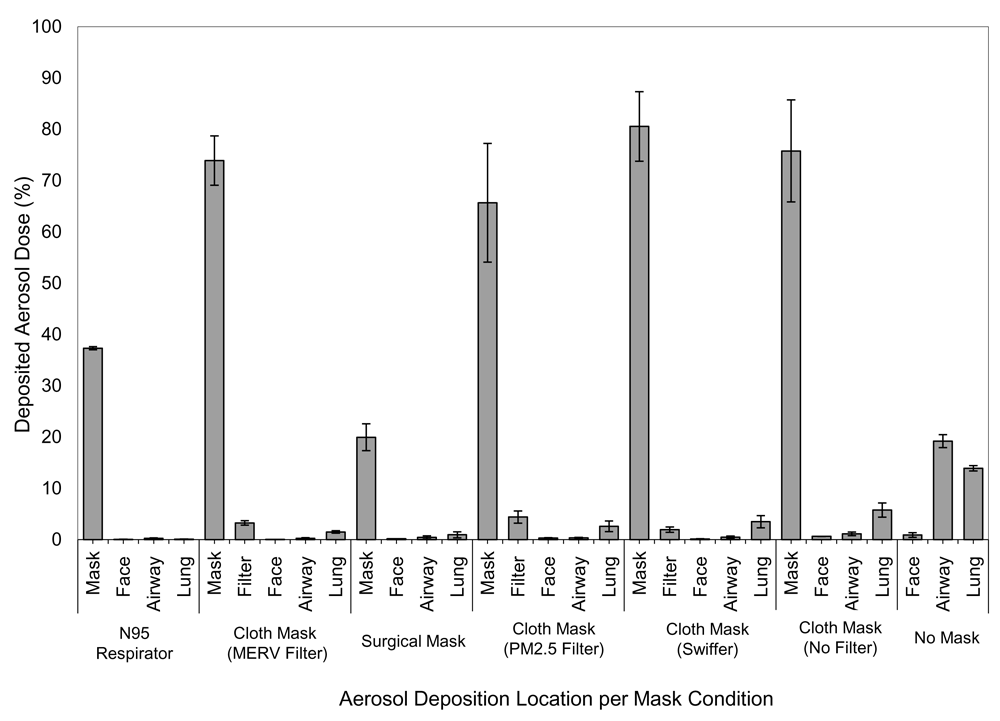
Data shown represent the mass of albuterol particles recovered from the face, airway, and lung with and without different masks. These data were then referenced to the nominal albuterol dose (5 mg) placed into the nebulizer as delivered dose %. Data are mean ± SD (standard deviation) with n=5 runs with each configuration and n=3 for N95. These descriptive data were not analyzed for statistical differences.
The major finding of this study was that a cloth mask, cloth mask/filter combination, surgical mask, and N95 respirator provide filtration of coarse aerosol particles capable of containing viral pathogens from reaching the face, airway and lungs when compared to no face covering. For the fine water vapor particles, also capable of transmitting infectious pathogens, the addition of a filter to a cloth mask or wearing an N95 respirator showed better protection than a cloth with no filter or surgical mask.
This study is one of the first to use realistic 3D printed airway models and a spontaneously breathing test lung to measure mask efficiency with aerosols. Although these findings are not generalizable to all face coverings currently recommended by the CDC, they do show benefit of wearing a cloth mask, cloth mask with filter, surgical mask and N95 respirator in protecting the wearer. It is suggested that the general public protect the critical supply of surgical masks and N95 respirators for healthcare workers and instead wear cloth masks. Based on these findings, integrating a filter into a cloth mask may decrease the number of both fine and coarse particle transmission.
Since the emergence of COVID-19, much of the public health messaging has promoted face coverings, when social distancing is challenging, to lower the spread of the virus. Previous studies have demonstrated advantages of mask wearing for source control. (Patel et al., 2016) In this in vitro study, we demonstrated that there may be some benefits of wearing facemasks as they filtered large particles and some effectively filtered tiny water vapor particles. Makison Booth et al. (2013) measured the permeability of surgical masks and their ability to protect the wearer from influenza bioaerosols. Their study, in vivo, suggested that the influenza virus can survive in particles that penetrated a surgical mask. Davies et al. (2013) demonstrated that both surgical mask and cloth masks reduce the total number of microorganisms expelled when coughing, however the surgical mask had superior performance especially with smaller particles. Our water vapor testing results align with a Li et al. (2006) study that showed surgical masks have significantly higher water vapor permeability than N95 respirators. Our study added to the body of literature, by testing cloth masks and filters in comparison with both the surgical mask and N95 respirator.
A limitation of this study is that the efficacy of filtration was measured with non-biologic particles. Despite this the effectiveness of the masks is best estimated on the measurement of blocking aerosols as essentially any aerosolized particles will behave similarly despite having different biologic properties. (Davies et al., 2013) While we considered fine and coarse inhaled particle transmission, not all particles penetrating masks in the community may be infective or be indicative of viral viability or exposure to the airway, lungs or of the virus. In addition, we were also unable to test the wide range of respirable aerosols that may be emitted by infectious individuals. Additionally, the study methods exposed the masks, airway and face directly to large amounts of inhaled particles. Findings from this comparative study should be approached with trepidation as this simulates a ‘worst case scenario’; wherein a potentially unmasked infected individual is speaking or coughing directly into direct proximity of an unmasked/masked individual. In most situations in the community, a mask wearer will not be exposed to infectious aerosols in such close proximity. Also, it is currently unsubstantiated in the literature what amount or location of inhaled virons leads to infection and what, if any, influence the amount and location of deposition of inhaled virus exposure impacts the disease severity. As we were unable to assess these important factors in the current study, future studies are needed in order to evaluate particle transmission and efficiency at different physical distances, smaller particle sizes, and different environmental conditions.
Another limitation of this study is that the N95 respirator was unable to be fit-tested to the 3D printed face. This may have led to some leaks around the masks. It would be beneficial to test the permeability of mask on research subjects with the ability to be fit-tested. The N95 testing was limited to three runs each, unlike five runs for other masks, due to regional supply shortages. Our study was also limited to one brand of N95 respirator, surgical and cloth mask. Further research into additional types of cloth face coverings as well as how wear time and different environmental conditions influence filtration also need to be considered.
This study does not suggest that a cloth mask alone or any mask will prevent the airborne transmission and spread of this virus. However, the addition of a filter to the cloth mask may improve the performance. The possible contribution of infective aerosols to the current pandemic suggests the advisability of wearing a suitable mask in combination with other measures such as social distancing and handwashing.
Figshare: Assessment of Mask Efficiency for Preventing Transmission of Airborne Illness Through Aerosols and Water Vapor. https://doi.org/10.6084/m9.figshare.14758497 (Ringer, 2021).
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| Gates Open Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Civil engineering, with large laboratory experience. Performed extensive mask performance measurement during the pandemic.
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Sickbert-Bennett E, Samet J, Clapp P, Chen H, et al.: Filtration Efficiency of Hospital Face Mask Alternatives Available for Use During the COVID-19 Pandemic. JAMA Internal Medicine. 2020; 180 (12). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Aerosol Scientist and Pulmonary Physiologist
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 20 Jul 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with Gates Open Research
Already registered? Sign in
If you are a previous or current Gates grant holder, sign up for information about developments, publishing and publications from Gates Open Research.
We'll keep you updated on any major new updates to Gates Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)